
Бесплатный фрагмент - Theory of three-electrone bond in the four works with brief comments
I express my deep gratitude to my son, Bezverkhniy Vitaliy Volodymyrovich, for participation in the development of the theory (some parts as a co-author), and for his invaluable contribution to the English translation.
1. Bezverkhniy V. D. Structure of the benzene molecule on the
basis of the three-electron bond.
http://vixra.org/pdf/1606.0152v1.pdf
2. Bezverkhniy V. D. Experimental confirmation of the existence of the three-electron bond and theoretical basis ot its existence.
http://vixra.org/pdf/1606.0151v1.pdf
3. Bezverkhniy V. D. A short analysis of chemical bonds.
http://vixra.org/pdf/1606.0149v2.pdf
4. Bezverkhniy V. D., Bezverkhniy V. V. Supplement to the theoretical justification of existence of the three-electron bond.
http://vixra.org/pdf/1606.0150v1.pdf
5. Bezverkhniy Volodymyr Dmytrovych (viXra):
http://vixra.org/author/bezverkhniy_volodymyr_dmytrovych
Note 1. Three-electrone bond it is an existing particle (object)
Three-electrone bond it is an existing bond, not a mathematical or physical model.
And if the three-electron bond exist, then:
1) We can represent the one true formule of benzene (p. 3 — 5 Structure of the benzene molecule on the basis of the three-electron bond. http://vixra.org/pdf/1606.0152v1.pdf).
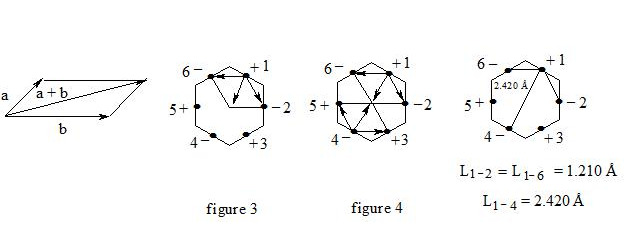
One of the drawbacks of the resonance theory is that resonance structures do not exist in reality, and their objectification is a mistake. And assuming the existence of three-electron bond, we can represent the real formula of benzene, aromatic compounds, carboxylate anion, ozone, oxygen, etc. (p. 19—29 Structure of the benzene molecule on the basis of the three-electron bond. http://vixra.org/pdf/1606.0152v1.pdf).
2) We can simply and clearly explain the increase in the multiplicity of benzene from 1.5 to 1.67. by MO method calculations give a value of 1.67, but Pauling from resonant structures, which is logical (2 and 4 of the electron) gave 1.5.
If the multiplicity is greater than 1.5 (eg 1.67), since the communication multiplicity in classical chemistry correlates with the amount of the bonding electrons (even if it is average) like:
2 electrons multiplicity 1;
4 electrons multiplicity 2;
6 electrons multiplicity 3;
thene in benzene at a multiplicity of 1.67 in six (6) aromatic bonds as it further appears 1 electron:
1.67 — 0.17 = 1.5
6 * 0.17 = 1.02
At the three-electron bond in benzene and interaction through a simple explanation of the cycle — the cycle just a little compressed.
3) We can check experimentally: if the three-electron bond and interaction through the cycle are real, then it logically follows the bending real chemical bond density in benzene into benzene.
It is important that the maximum density of the chemical bond will be shifted to the center of the benzene cycle link, which is what we are seeing in the atomic force microscopy images (AFM) pentacene (p. 1—2 Experimental confirmation of the existence of the three-electron bond and theoretical basis of its existence. http://vixra.org/pdf/1606.0151v1.pdf).
4) Experimental predicted effects: anti-aromatic system (core system) should be flat in order to make it through the interaction cycle. Therefore, to obtain photos and AFM antiaromatic cyclobutadiene cyclooctatetraene must be on a special matrix to consolidate their atoms to make the system perfect planarity (to make it through the interaction cycle), and after that, take a picture AFM permission. And if anti-aromatic photo is received, then we should see a shift of three-electron bonds outside the cycle, and, the picture will be in pentatsene but the loop (p. 4–5 Experimental confirmation of the existence of the three-electron bond and theoretical basis of its existence. http://vixra.org/pdf/1606.0151v1.pdf).
And if think … … reflect the existence of three-electron bond directly from the theory of resonance (resonance structures do not exist, in reality there is something average between them — and now think that should really be the basis of this, some real structure?.. of course the three-electron bond!!!).
The three-electron theory of communication accepted for granted the existence of three-electron bonds (one axiom), everything else is derived logically.
The need to introduce three electron bond in the description of the benzene molecule can be understood (to some extent) reading the book (Chapter IX «Chemistry») Loren R. Graham. Science, Philosophy, and Human Behavior in the Soviet Union, Columbia University Press, 1987.
Short and interesting in Chapter IX «chemistry» of this book Loren R. Graham describes the concept of resonance theory in chemistry (description of the benzene molecule), as well as its criticism of the Soviet period.
Loren R. Graham — Professor at MIT (USA) on the big Material of actually analyzes full of dramatic story of the interaction of dialectical materialism and Soviet scients in the period from 1917 to mid-80s.
Provides a links to the original works.
Here is a quote Pauling:
«We can say… that the molecule can not be satisfactorily represented by any particular structure of the valence bond and stop trying to tie its structure and properties of the structure and properties of other molecules. But, using valence bond structures as a basis for discussion, we are using the concept of resonance can give an explanation of the properties of the molecule, directly and simply in terms of other properties of the molecules. For us, convenient, for practical reasons, talk about the resonance of molecules among several electronic structures.»
Here’s another quote Academician Koptyuga:
British journalist: «If you look at the history of science after the Revolution, you will see several cases of political interference in the fundamental research… What do you think, could this happen again?»
Academician V. Koptiug, Chairman of the Siberian Branch of the USSR: «You see, this is a very complex issue…
When in the past with philosophical positions criticized the concept of resonance in chemistry… is, from my point of view, it is true.
But when a general philosophical position of trying to solve major scientific problems, such as whether genetics science or pseudoscience, it was a mistake.»
TV interview BBC, November 8, 1981.
Who loves the history of chemistry (of benzene) is very interesting and informative.
«Now the question is how to explain the existence of the three-electron bond in benzene and other molecules and ions from the point of view of quantum theory. It stands to reason that any placement of three electrons on the same atomic or molecular orbital is out of the question. Therefore it is necessary to lay the existence of three-electron bond in molecules in reality as an axiom. In this case the three-electron bond in benzene can be actually considered a semi-virtual particle. A real particle, such as an electron, exists in the real world for indefinitely long time. Virtual particles exist for the time which is insufficient for experimental registration (strong interactions in atomic nuclei). So we shall call the three- electron bond which really exists for indefinitely long time only in molecules and ions a semi-virtual particle.
The three-electron bond as a semi-virtual particle has certain characteristics:
its mass is equal to three electronic masses,
its charge is equal to three electronic charges,
it has half-integer spin (plus, minus 1/2)
and a real spatial extension.
That is, our semi-virtual particle (the three-electron bond) is a typical fermion.
Fermions are particles with half-integer spin; they follow the Fermi-Dirac statistics, and have appropriate consequences, such as the Pauli exclusion principle etc. An electron is a typical fermion, and therefore such distribution in atomic and molecular orbitals is accepted (calculated). It follows that the three-electron bond in benzene is a real fermion in benzene, so quantum calculations can be extended to the molecule of benzene (and other systems) with the use of corresponding fermion (i.e. three-electron bond as a particle) instead of the electron in calculations. Then everything shall be made as usual: the Pauli exclusion principle, distribution in MO, binding and disintegrating MO, etc.»
(p. 4–5 Experimental confirmation of the existence of the three-electron bond and theoretical basis of its existence. http://vixra.org/pdf/1606.0151v1.pdf).
Note 2. Chemical bond — it is the interaction of fermions
Following from the above, interaction of two three-electron bonds in benzene (or rather interaction of three pairs) through the cycle is a typical interaction between two fermions in a molecule at a distance of 2.4 Å which is similar to the interaction of two electrons at the chemical bond formation.
Бесплатный фрагмент закончился.
Купите книгу, чтобы продолжить чтение.
