
Бесплатный фрагмент - Pediatric stroke
Revascularization and reconstructive surgery in children with cerebrovascular disease
Writing Team
Olga Benuanovna Belousova
Neurologist, Dr. Med. Sci., leading research scientist in the Clinical Vascular Neurosurgery Department of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Anton Evgenyevich Korshunov
Neurosurgeon, Cand. Med. Sci., senior research scientist in the Clinical Pediatric Department of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Irina Andreyevna Nagorskaya
Medical Psychologist, Cand. Psych. Sci., Medical Psychologist in the Mental Research Team of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Vasiliy Andreyevich Lukshin
Neurosurgeon, Cand. Med. Sci., senior research scientist in the Clinical Vascular Neurosurgery Department of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Olga Aleksandrovna Lvova
Pediatric Neurologist, Dr. Med. Sci., assistant professor in Chair of Psychiatry, FSBEI of Higher Professional Education «Urals State Medical University» of the Ministry of Healthcare of the Russian Federation, leading research scientist in the Laboratory of Brain and Neurocognitive Development of FSAEI of Higher Professional Education «Ural Federal University named after the first President of Russia B.N. Yeltsin»
Olga Borisovna Sazonova
Neurophysiologist, Cand. Med. Sci., leading research scientist in the Laboratory of Clinical Neurophysiology of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Elena Viktorovna Shevchenko
Neurosurgeon, Cand. Med. Sci., junior research scientist in the Clinical Vascular Neurosurgery Department of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Lyudmila Valentinovna Shishkina
Pathomorphologist, Cand. Med. Sci., Head of Laboratory of Pathomorphology in FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
Dmitry Yuryevich Usachev
Neurosurgeon, Corresponding Member of the Russian Academy of Sciences, Dr. Med. Sci.,Prof., Deputy Director for Science of FSAI «Burdenko Neurosurgery Institute» of the Ministry of Healthcare of the Russian Federation
List of acronyms and conventional symbols
ABP — arterial blood pressure
ACA — anterior cerebral artery
ACVD — acute cerebrovascular disease
ADHD — attention deficiency and hyperactivity
disorder
ADP test — adenosine diphosphate induced
platelet aggregation test
AHT — arterial hypertension
ASA — acetylsalicylic acid
ASL — Arterial Spin Labeled
ASPI test — arachidonic acid induced
platelet aggregation test
BCA — brachiocephalic arteries
CAG — cerebral angiography
CCA — common carotid artery
CCVD — complete cerebrovascular disease /
complete stroke
CN — cerebral nerves
CNS — central nervous system
CO — cerebral oximetry
CPISR — Canadian Pediatric Ischemic Stroke Registry
CVD — cerebrovascular disease
CVS — cardiovascular system
DEP — dyscirculatory encephalopathy
DMB — dura mater of brain
ECA — external carotid artery
ECG — electrocardiography
EchoCG — echo-cardiography
EDAS — encephalo-duro-arterio-synangiosis
EDMS — encephalo-duro-myo-synangiosis
EEG — electro-encephalography
EICMA — extra-intracranial microvascular
anastomosis
EMS — encephalo-myo-synangiosis
ICA — internal carotid artery
INR — international normalized ratio
IS (AIS) — ischemic stroke (arterial ischemic stroke)
LBFR — linear blood flow rate
MASGS — Modified Ashworth Scale of
Grading Spasticity
MCA — middle cerebral artery
MONICA — The World Health Organization’s
Multinational Monitoring of Trends and
Determinants in Cardiovascular Disease
MRA — magnetic resonance angiography
MRI — magnetic resonance imaging
NIHSS — National Institutes of Health Stroke Scale
NSA — National Stroke Association
OA — occipital artery
PCA — posterior cerebral artery
PComA — posterior communicating artery
PET — positron emission tomography
PS — pial synangiosis
SCT AG — spiral computed angiography
SCT or CT — spiral computer tomography
STA — superficial temporal artery
TCUSDG — transcranial ultrasonic dopplerography
TIA — transitory ischemic attacks
US — ultrasonography
VBS — vertebrobasilar system
WHO — World Health Organization
Introduction
Pediatric stroke. Revascularization and
reconstructive surgery in children
Pediatric stroke is one of the most widely discussed problems in contemporary medicine. This is, primarily, associated with the fact that a cerebral vascular disease (CVD) is considerably less common in childhood than in adults, and, therefore, less known. At the same time, children, who had suffered CVD, constitute an essential group among disabled children. This determines the need for a closer study of the pediatric stroke problem, particularly, in the background of successful conservative and surgical treatment of strokes in adult population.
The spectrum of clinical manifestations of the pediatric stroke is wide enough — from mild focal and isolated general cerebral symptoms to the formation of a significant neurologic deficiency with a predisposition to recurrence with the subsequent sustained disability and a high risk of fatality. Thanks to a widespread distribution and technical improvement of neuroimaging methods, the pediatric stroke is diagnosed with the ever increasing frequency. Nevertheless, the low awareness of neurologists about the CVD problem in childhood, including the transitory ischemic attacks (TIAs), frequently leads to difficulties in diagnostics and, consequently, to delayed and insufficient medical aid. Due to the variety of reasons, clinical manifestations and the course of the pediatric stroke, selecting the patient management approach becomes difficult, especially, in neurology and brain surgery departments in small city hospitals, where medical specialists lack sufficient experience in treatment of this disease.
This book presents basic literature data on etiology, pathogenesis, clinical manifestations of a pediatric stroke, examination methods and approach to the conservative and surgical treatment of acute and chronic cerebral ischemia in children as well as our own studies on diagnostics and results of conservative and surgical treatment of children with the disease onset at the age of the 1-st day of life up to 18 years old, all obtained on the basis of Burdenko Neurosurgery Institute and FSBEI of Higher Professional Education «Urals State Medical University» of the Ministry of Healthcare of the Russian Federation.
This book will enable a wide range of pediatric specialists to get an idea about the specific features of a pediatric ischemic stroke, its diagnostics, conservative treatment principles and options for surgical treatment of this disease.
Chapter I.
Pediatric stroke. General information
Nowadays, the mortality rate of cerebrovascular diseases in Russia is one of the world’s highest. A cerebrovascular disease holds one of the first places among the most frequent mortality and disability causes, just as in economically developed countries too. The World Health Organization’s Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) determines the course of a stroke as «a sudden neurologic deficiency sustaining for over 24 hours, or a sudden death». This definition includes both ischemic and hemorrhagic strokes [262].
In a pediatric population, an ischemic stroke is a less frequent pathology as compared to the adult population. Strokes occur in children of any age [33]. Delayed or erroneous diagnosis of a stroke in children still remains a common enough event [80; 104].
The descriptions of individual clinical cases of cerebrovascular diseases in children can be found in literature since the 17-th century. The first description of a stroke in a child is considered to be made by T. Willis in 1667. J. Wepfer (1658) mentioned sick children, who had a hemiplegia, which was emerging and regressing within a day or faster [278]. The disease termed as «an infantile hemiplegia» was presented in the works by W. Osler (1889), B. Sachs and F. Peterson (1890) as well as S. Freud (1893) in a series of pediatric patients, who had suffered a stroke. It was only in 1927 that F. Ford and A. Schaffer published the first ever systematized description of methods for assessment and treatment of children with ischemic strokes. The authors analyzed the etiology of a pediatric stroke as well as the methods and the results of treatment, which had a subsequent effect on the quality of life [98]. V. Hachinski (1982) described non-specific symptoms, such as a headache and syncopes [118]. It is important to note that many problems outlined by them still remain pertinent even today.
The works by M. Norman (1957), C. Fischer (1959), E. Frantzen (1961), E. Bickertaff (1964), J. Jackson (1970), J. Abraham (1971), W. Kannel (1972) about pediatric strokes are, doubtless, interesting, although these publications did not contain any mentioning of the transient cerebrovascular diseases in childhood [131]. In later studies, the transient cerebrovascular diseases, or, in other terms, the transitory ischemic attacks (TIAs), were noted to occur in children much more frequently than strokes [209]. In 2006 G. Ganesan et al. published an article on the results of a retrospective (from 1978 to 1990) and prospective (from 1990 to 2000) survey of children, who had suffered a stroke, with the use of a neuroimaging. They described 212 patients, including 97 ones with an erroneous initial diagnosis. 79 children were noted to have a growing neurologic deficiency (29 strokes, 46 TIAs, 4 fatal cases due to a recurrent stroke), while during the analysis of the subsequent 5 years 51 children (67%) were noted to have recurrent episodes of cerebrovascular diseases [104].
The number of publications on the subject of a pediatric stroke is growing worldwide with every year. In recent years, educational seminars and topical sessions on this problem appeared in the European Stroke Organization Congress program (Nice, 2014; Glasgow, 2015; Barcelona, 2016). Nowadays, practitioners working abroad can be guided by two manuals: an American one — «Management of Stroke in Infants and Children» released in 2008 and a European one — «Stroke and cerebrovascular disease in childhood» published in 2011 in London [105; 267]. Thus, practical manuals accumulating the results of scientific research and permitting to make clinical decisions are solitary and rarely updated.
The lack of universally accepted international recommendations or guidelines hampers the choice of approaches to treatment and prevention of pediatric strokes. A low awareness of pediatric neurologists on the problem of pediatric strokes and TIAs often leads to difficulties in diagnostics and inadequate therapy of pediatric patients and, therefore, to a delayed and inadequate care, which was noted by V.P. Zykov (2008), F. Kirkham (2011) and A. Mallick (2014) in their works. The main drawback of the research studies presented in literature consists in the fact that only some individual states were specified as those relevant to risks, which, as a rule, was determined by the specialty of a research team (infectologists, rheumatologists, geneticists, hematologists, etc.). The paucity of assessed sampling children in these studies and the restriction of data acquisition to a specific age group (infants, teenagers, etc.) hampered the potential generalization of results obtained within the boundaries of all age groups.
1. Epidemiology
The rate of strokes in the adult population of the Russian Federation is about 500,000 cases per annum, the average stroke morbidity rate is 4.6 incidents per 1,000 of the population annually (the NSA data, 2003).
According to literature data, the average global pediatric stroke morbidity rate varies within a range of 0.93 — 13 incidents per 100,000 of the population annually [33]. According to data of the American Heart Association & American Stroke Association, 2012, the highest stroke incidence rate is noted during the first year of life — approximately 1 incident per 4,000 liveborns [219]. The stroke morbidity rate in children aged from 0 to 15 years in the USA is 6.4 incidents per 100,000 children [222]. During the last 10 years, this number remained stable, but the recent research showed that the incidence rate was 3—4 times higher, than it had been stated earlier [39].
M. Giroud et al. (France, 1995) report that in children below 16 years old the CVD incidence rate reaches 13 incidents per 100,000 children annually. This number includes 7.9 cases of ischemic CVD per 100,000 children and 5.1 cases of hemorrhagic CVD [110].
According to data of the Canadian Pediatric Ischemic Stroke Registry (CPISR), in 2000 this parameter was recorded at the level of 2.7 incidents per 100,000 annually [160]. F. Kirkham et al. reported that in 2004 the incidence rate of CVD in British children was 13 incidents per 100,000 of pediatric population [143].
Thus, according to various data, the morbidity rate of pediatric hemorrhagic stroke at the age from 1 month to 18 years varies from 1.5 to 5.1 (on the average, 2.9) and that of the ischemic stroke — from 0.6 to 7.9 incidents per 100,000 of the population annually. Also, there are some data on the percentage ratio of these two types of strokes: 55% is accounted for ischemic strokes and 45% — for hemorrhagic ones [77]. At the same time, in newborns this parameter is considerably higher for both types of CVD: 6.7 and 17.8 per 100,000 of the population annually for hemorrhagic and ischemic types of CVD respectively [11; 167; 215].
These figures show that, generally, the ischemic stroke incidence rate in children is higher than the hemorrhagic one, although this difference is not as big as in adults [8; 33; 40], which makes the CVD structure in children essentially different.
During the period from 1979 till 1998, in the USA the child mortality decreased by 58%. Such a decrease is deemed to occur due to the improvement of the treatment quality, not due to the drop in stroke morbidity rate [149]. According to latest data, from 20% to 40% of children die after the strokes in the USA [219], and the stroke is among ten leading causes of child mortality [68]; about 3,000 children and teenagers (below 18 years old) suffered a stroke in 2004 [149]; during the period from birth to an age of 18 years old the stroke risk is almost 11 incidents per 100,000 children annually [219]; strokes occur in boys approximately 1.3 times more frequently than in girls [222]; in Afro-American children the stroke risk is higher than in children from Europe and Asia [222].
The stroke mortality in children varies from 7% to 28% [100; 161], being higher in hemorrhagic strokes (up to 40%) than in ischemic ones (8—16%). The fatal outcome usually occurs in the early rehabilitation period, which is deemed to be the most dangerous time for recurring acute vascular episodes and patient death. Such indicators in children with ACVD are, generally, consistent with the statistical data on adults. However, the mortality level of 10%-40% may be regarded as the highest-ever for pediatric practice, which permits to determine strokes in this age group as one of the emergency pathologies threatening with fatal impairment of vital functions.
The official statistics on pediatric stroke morbidity rate in our country are not available [33]. In literature there are some data for individual areas or institutions. Specifically, based on an example with one of the central regions of Russia, V.M. Delyagin et al. (FSI «Federal Research & Clinical Centre of Pediatric Hematology, Oncology and Immunology», Moscow) reported that during the period from 2006 to 2009 the ACVD morbidity rate among children (excluding newborns) was from 0.93 to 1.1 incidents per 100,000 children annually. When estimating the number of children and teenagers with strokes per total number of children taken to multidisciplinary children’s hospitals, the stroke incidence rate is 3.5 per 1,000 patients annually [7; 11], which corresponds to data of foreign multi-center surveys. This number includes 2.8 children aged from 0 to 11 years old per 1,000 patients annually and 0.7 children aged from 12 to 17 years old (teenagers) per 1,000 annually; the average pediatric stroke morbidity rate (from 1 month to 18 years old) is about 8 incidents per 100,000 of population annually. The mortality among children with CVD reaches 0.6 per 100,000 of population annually [8].
Having analyzed the data on 143 patients aged from 0 to 17 years old with CVD (the age median of 5 years old, in 68 boys (48%) and 75 girls (52%)), the same authors concluded that all types of CVD occur equally often both in boys and girls, with the exception of TIAs, which are recorded in girls three times more frequently than in boys. The authors also note a high percentage (13%) of recurrent strokes in children, while the highest risk of a recurrent CVD is recorded during the first 2 weeks after the disease onset [8].
According to data of the Emergency Call Service of Moscow, in 2012 there were 157 ambulance responses on calls to children and teenagers with the ACVD diagnosis, and in 2013 — 179 [4].
Employees of the FSBEI of Higher Professional Education «Urals State Medical University» analyzed the stroke in children living in the area of Yekaterinburg (with population of 1.5 million people) and Sverdlovsk region (4.5 million people). The following parameters were assessed: the stroke registration rate in the years from 1995 till 2015; the morbidity rate during the last five years, including children of the first year of life; gender distribution characteristics; incidence rate of fatal outcomes and recurrences in 162 children with IS and 73 children with TIA.
The study was held for 10 years. During this period, the information was distributed among the pediatric neurologists of the city and the region, who actively referred already followed-up and new pediatric patients with diagnosed or suspected ischemic ACVD to hospitals. We suppose that practically all patients with the onset of IS or TIA occurring in childhood were included into this database, and this permits to regard this study as an epidemiological survey.
According to the data obtained, during the last five years the morbidity rate was: 3.4 (2011), 4.9 (2012), 4.9 (2013), 4.6 (2014) and 5.0 (2015) per 100,000 of the pediatric population annually. Fig. 1 shows the total number of registered children with strokes on a specified territory during the last 20 years since 1995, when neuroimaging (brain CT) and emergency diagnosing became possible.
It must be emphasized that the obtained indicators are closer to the lower threshold of values stated in literature (2—26.7 per 100,000 annually). At the same time, a distinct tendency for growing stroke registration incidence rate in children in the surveyed area, which can be observed during the last ten year period in all countries, where ACVD morbidity is registered among children.
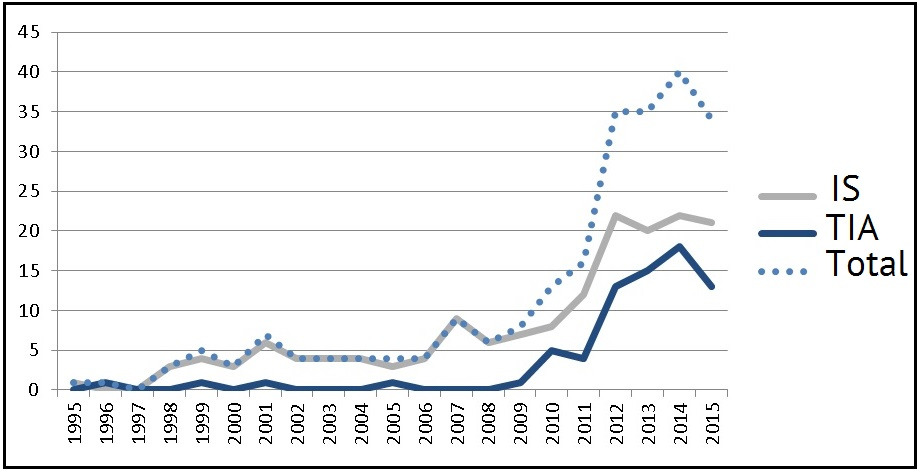
The average age of children with IS manifestation at the age of below one year was 19.5±1.2 weeks (we revealed 7 infants with fetal / perinatal onset of IS) and at the age of above one year — 6.2±0.4 years. For TIA this parameter was 11.8±0.3 years.
The gender distribution of patients was even, and matched the literature data: boys with IS constituted 62.7% (n=102), and boys with TIA — 45.2% (n=33).
Based on literature data, the average risk of recurrent strokes in children is 20%, while in children with a single revealed risk this parameter is within 8%, and in children with a combination of two or more risks it grows at an exponential rate and reaches 42% [145; 148].
According to data of the FSBEI of Higher Professional Education «Urals State Medical University», the recurrence is also recorded on the levels of 14.2% (n=23) and 70.4% (n=50) for IS and TIA respectively. The average incidence rate of recurrent ISs was 1.6±1.1 (1–2 episodes of IS compared to 2–19 incidents of TIA), the average incidence rate of TIA was 3.4±0.5 incidents (from 2 to 20 episodes). It is the low level of ACVD detectability in childhood, which is supposed to cause the lack of timely and comprehensive examination, correct diagnosing and timely application of secondary prevention measures. For example, there was a patient registered, who had suffered 6 TIAs and 2 ISs, before he was subjected to a comprehensive examination, which diagnosed the moya-moya disease.
The disability status was given to 61.2% (n=90) and 9.1% (n=4) of patients from 125 and 62 children with IS and TIAs respectively, whose catamnesis was known. It should be noted that the disability in the group of children with TIAs was caused by a non-neurologic deficiency: two children had an acknowledged moya-moya disease, one had a chronic renal insufficiency, and one — a congenital heart defect.
The mortality in a group of children with IS was 3.3% (n=4, 2 boys and 2 girls); all the patients, who had suffered TIAs, were alive by the moment of the last follow-up visit (minimum 2 years of follow-up).
Thus, the literature data and the results of limited epidemiological surveys in Russia permit to conclude that ischemic strokes are a relatively rare disease in pediatric practice, although they are characterized by a high rate of recurrence, disability and mortality.
2. Pediatric stroke classifications
As already stated above, the ratio between hemorrhagic and ischemic strokes in children essentially differs from that in an adult age group. There is no unanimous opinion on the ratio between these variants in children. Apparently, the prevalence of an ischemic or a hemorrhagic ACVD variant in every new survey is associated with the specialization profile and medical care type in a healthcare facility.
Also, there are discrepancies in determination of a stroke variant in a child. For instance, the national research community failed to agree whether periventricular ischemia as well as intraventricular and subarachnoid hemorrhages can, by way of a morphological substrate of perinatal impairment of infants’ nervous systems, be considered to be equivalents of ischemic or hemorrhagic ACVDs (by analogy with adult patients). Authors of foreign clinical manuals on diagnostics and treatment of strokes say that they excluded infants with such lesions from analyzed literature sources. Based on provided epidemiological indicators, it also becomes evident that the researchers did not include patients with perinatal encephalopathy into their analysis scope [183; 107; 146; 220; 280].
It is well known that an ischemic stroke is broken up into the following categories:
• complete stroke — a cerebrovascular disease, which results in the formation of a sustained neurologic deficiency; ischemic lesions of cerebral tissue are detected by spiral computed tomography (CT) and magnetic resonance imaging (MRI) of brain;
• minor stroke — an acute development of a neurologic deficiency with subsequent complete regression within 2—3 weeks; small ischemic lesions may be detected by CT and MRI of brain;
• evolving stroke or stroke in evolution — an acute development of cerebral ischemia accompanied by a gradual growth of the neurologic deficiency during several days.
A separate nosological form of ACVD is transitory ischemic attacks (TIAs), which are characterized by a sudden development of a neurologic or retinal deficiency of ischemic nature, which is related to a specific artery territory and which regresses completely within 24 hours. TIAs occur considerably more frequently than strokes. Regarding their incidence rate, TIAs are subdivided into rare (1—2 times per year), mid-frequent (3—6 times per year) and frequent (once per month or more frequently) [17]. TIAs may be a manifestation of a chronic cerebral ischemia (insufficiency) with a high risk of subsequent development of a massive IS. Thus, a TIA may be considered an antecedent of IS.
The international terminology used for describing a pediatric stroke includes the following notions:
1. fetal (prenatal, intra-uterine) stroke — before the child birth;
2. perinatal stroke (when the disease develops during the period from the 28-th gestational week till the end of the first month following the birth);
3. pediatric stroke — at the age of 1 month following the birth until 18 years old [37; 148].
Presently, in children it is proposed to single out the following pathogenetic types of an ischemic stroke: hemodynamic, metabolic, embolic and occlusive.
There is no generally accepted and acknowledged by all specialists classification of CVDs in children. Above, we have presented a classification, which considers the age, when the stroke onset occurred.
Regarding the periodization of the disease itself, national specialists prefer to rely on the time frames formed in adult practice.
Groups of experts attempt to propose the pathogenetic variants of a pediatric stroke classification, yet they fail to end the discussions. For instance, they propose an anatomical classification named CASCADE (Childhood AIS Standardized Classification and Diagnostic Evaluation), which considers the localization and/or source of thrombosis/embolism of brain arteries (minor cerebral arteries, major cerebral arteries, aorta and cervical arteries, heart) [57]. The development of CASCADE classification was aimed at creating an analog of TOAST (Trial of ORG 10172 in Acute Stroke Treatment) accepted in adult practice, which was practically achieved [76]. However, the criteria specified in it can be hardly met or, in fact, cannot be met at all: e.g., they imply a histological acknowledgement of changes in cerebral vessels. Another essential drawback of this classification is deemed to be ignoring the embolic variant of IS and thrombophilic states in it.
3. Aetiopathogenesis and risks
A pediatric stroke is heterogeneous in aetiopathogenesis. If in adults strokes are associated most frequently with atherosclerosis of brachiocephalic arteries (BCA) [26; 27], the etiology of strokes in children is diverse and complex [40]. In literature there is a quite exhaustive list of diseases and syndromes, which are fraught with a risk of cerebral ischemia in childhood, adolescence and youth. According to data of the American Heart Association & American Stroke Association (2012), half of all children, who had suffered a stroke, had risks [219].
Complexity and diversity of etiology imply a wide circle of specialists, who must keep an alert eye on strokes in their routine practice.
Heart diseases (congenital and acquired) present one of the most significant risks equal to about 20—30% of the causes of ischemic strokes in childhood [161]. A combination of left-heart embolisms (or paradoxical embolism in right-to-left cardiac shunt) and cardiac decompensation is important in pathogenesis of cardioembolic variant of an ischemic stroke [1; 7; 22; 52]. There is a description of cases of paradoxical embolism into the cerebral vessels of children and young people in the background of an atrial septal defect, open foramen ovale, in cases of arteriovenous malformations of pulmonary vessels and neurocutaneous syndromes [280]. While this problem was in focus, the attention was again attracted to minor cardiac abnormalities. Regarding patients with vague etiology of stroke, it is, primarily, recommended to rule out sources of hidden or paradoxical embolism as an open foramen ovale, a mitral valve prolapse and an atrial septal aneurysm [1; 52; 56; 38]. The literature data state that imaging reveals «silent» brain infarctions in 25% of patients with mitral stenosis. Also, clinically «silent» ischemic lesions of brain tissue are found in 20% of newborns with heart diseases on a pre-surgery stage and in 17.4% — on a post-surgery stage [63; 181; 221]. Presently, several studies were held with the attempted prognostication of strokes in infants with congenital heart diseases. A significant role of duration and an intensity of hypoxia in newborns, resuscitation procedures, prematurity and duration of waiting for surgical intervention were indicated as ACVD risks at pre-surgery and post-surgery stages [62; 112; 161; 181; 183; 210].
Cardiac arrhythmias are considered to be a very rare cause of strokes in childhood and youth, as opposed to adults. Nevertheless, it should be kept in mind regarding children with hyperthyreosis, rheumatic heart diseases, after surgical interventions and in the structure of Kearns-Sayre syndrome.
Cardiac myopathy as a manifestation of systemic diseases occurs in congenital myopathies (Duchenne, Becker, Emery-Dreifuss, etc.), Friedrich’s ataxia, mitochondrial diseases. With this pathology, both embologenic and hemodynamic variants of an ischemic stroke are possible. In some cases, a myocardial infarction and a stroke may develop simultaneously, which points at the similarity of pathogenetic processes leading to inadequate perfusion [7].
Hypercoagulation states are presently considered to be the most common causes of ischemic strokes in childhood — their contribution reaches 87% [3; 16; 138; 144; 170; 265]. However, a universally acknowledged screening protocol for thrombophilic state in a child with CVD has not been developed yet, and some researchers dispute the role of multigenic thrombophilias as risks of strokes and TIAs in children [82; 121; 142; 184; 194; 282].
During the last decade a large number of thrombophilic mononucleotide genic polymorphisms were described. Carriership of proaccelerin, prothrombin, plasminogen activator inhibitor and fibrinogen is considered to be most significant clinically [61; 138]. F5 genotypes: 1691 G> A and AA (Leyden mutation) as well as F2: 20210 G> A and AA are currently the only ones, whose prothrombotic effect is acknowledged in newborns. Besides, there is a good reason to suppose that with the carriership of thrombogenic mutations and polymorphisms in children the risk degree differs according to their age [5; 56; 138].
Presently, there are no doubts regarding the connection of hyperhomocysteinemia and MTHFR677C> T mutation with cerebrovascular and cardiovascular diseases [5; 16; 61; 280]. Homocystein acts as a prothrombotic factor due to activation of coagulation factors XII and V, increase in tissue factor expression and suppression of thrombomodulin expression. Besides, the rise of homocystein in blood leads to vascular endothelium damages, which reveals in neurotoxic and proatherosclerotic effects and contributes to the emergence of resistance to activated protein C [3; 31; 66; 267; 276; 290]. During the latest years, the role of hyperhomocysteinemia in damaging the vascular walls was proven as well as its prothrombotic and pro-atherosclerotic effects and its effect on the vasomotoric regulation [3; 32; 290].
The MTHFR enzyme gene provides for homocystein metabolism with the participation of a folic acid. The greatest practical significance belongs to a mononucleotide replacement of cytosine with thymine at position 677 of gene, thus leading to a replacement of alanine amino acid residue with valine in the catalytic core of methylene tetra hydro folate reductase enzyme (MTHFR). Individuals, homozygous by this allelic mutation, display a decrease of the enzyme activity by 60—70%, and heterozygous — by 35% [28; 159; 275]. It should be noted that all available data concern either fundamental aspects of the pathology study, or the population of adult patients.
According to literature data, children are noted to have a positive correlation relationship between the incidence rate of strokes (especially, in boys) and C677T polymorphism. The combination of several variants of genes is accompanied by a progressive rise of homocystein level in blood, and it increases the risk of a CVD [61; 142; 229; 255; 269; 289]. At the same time, a series of genic polymorphisms controlling the folate cycle activity showed their protective function regarding the cerebrovascular pathology in Asian and European populations [82; 142]. Despite the indirect relationship between pheno- and genotype of hyperhomocysteinemia, the researchers’ opinion is unanimous: determination of the homocystein level and the state of folate cycle genes must become an insatiable component of diagnostic suite in this group of patients, especially, in boys (class II, recommendation level B) [280].
In the literature there is a description of singular clinical cases of BCA thrombosis in children. There are no general statistics on CCA, ICA and MCA occlusions in pediatric population neither in national, nor in foreign literature. In 1951 M. Fisher was the first to describe the post-thrombotic occlusion formation steps in ICA of adults with hemodynamic and embolic mechanisms of an ischemic stroke, having analyzed the angiograms of patients with CVDs [96]. Post-thrombotic occlusions of major arteries in the heads and necks of children account for from 13 to 37% in the structure of CVD causes.
In childhood there are more than enough initiating agents capable of worsening the hemorheologic situation or decreasing the athrombogenic properties of a vascular wall. The adverse course of the post-natal adaptation period, infection, microtraumatization and metabolic disorders can act as triggering factors and lead to an acute cerebral ischemia in the background of the carriership of mononucleotide polymorphisms in thrombophilic spectrum genes and in the genes, which control the activity of folate cycle enzymes. In children with CVDs (especially, when the onset is in the perinatal period), it is recommended to search for prothrombotic mutations even, when other causes of ACVD are identified (class IIa, recommendation level C) [107; 280]. At the same time, the detection of thrombophilic polymorphisms is not an absolute factor inevitably leading to thromboses. Attention should be paid to the quantity of revealed mononucleotide mutations, the fact of homozygous carriership, the variants of genes — genic combinations and their phenotypic manifestations [5; 121; 286].
If the researchers have no doubts about hyper-homocysteinemia presenting a risk of thrombi formation at a non-typical age, the atherothrombotic variant of CVD is a casuistry for pediatric practice. Such variants are described in singular cases and associated with proven, genetically determined, dyslipidemic syndromes [165; 167; 189], most of which proceed asymptomatically [54]. Nevertheless, the selective screening and the lipid metabolism monitoring are recommended for patients with a family history of an early onset of vascular diseases and for the ones with an unclear cause of the stroke [111; 239].
The significance of the infection process as the releasing factor with the developing acute cerebrovascular insufficiency in the background is great both in newborns (up to 17.6% among all causes) and in elder patients (up to 40.7%) [60; 63; 223; 226; 280]. The clinical study showed the significance of a short duration (up to 4 weeks) and the fact of minor infections as an independent risk, which both enhance the probability of an ischemic ACVD 4.6 times as much [43; 240]. When analyzing the strokes of an unknown etiology, it was noted that shortly before the stroke children had varicella 3 times more often than in the population; the probability of a stroke also remained high within the first four months after varicella [157; 160]. Besides, the study held in 2006 showed that, in children with the earlier revealed immunodeficiency, the risk of the recurring stroke grew 20.9 times as much and correlated directly with the level of white blood cells during the acute period of the disease. A chronic infection and the immunodeficiency are supposed to make their own contribution to the development of recurrent incidents of CVD in children in the same way, as in adults [108].
It cannot be ruled out that an infectious process flows in the nervous system by the mechanism of vasculitis. Presently, the VIPS study (The vascular effects of infection in Pediatric Stroke Study) is held with the hypothesis stating that the presence of an infectious agent triggers an endothelial dysfunction, a systemic inflammatory process, which, combined with insufficiency of connective tissue and prothrombotic readiness, leads to damages of the vascular wall, its dissection, thrombogenesis, luminal occlusion and cerebral ischemia [13; 101; 127]. Later on, an embolism may occur from the artery dissection site as well as the hemorrhagic transformation of an ischemic lesion [13; 16; 101]. Also, apart from vasculitis, some hemorrhagic complications caused by coagulopathy may occur in severe somatic diseases [54; 55]. Presently, there are no such distinct diagnostic criteria of cerebral vasculitis in children, which could provide grounds to assert with confidence that it was this disease that had caused the CVD [13]. It is proposed to keep in mind that vasculopathy may be the most probable etiology of a CVD in all cases of TIA, and that it happens always in pediatric or young patients, especially, in the absence of evident risks [22; 55; 168].
During their early period of life, in children with CVD one cannot ignore the pathological course of the prenatal period, which could act as one of the initiating agents [105; 280]. Hemoreologic situation deterioration, arterial blood pressure measurement, endothelial dysfunction, systemic inflammatory response and other pathological processes triggered by a perinatally caused hypoxia initiate a hypercoagulation state and a thrombosis of various localization. In its own turn, the cascade microthrombogenesis mechanism affects the perfusion situation at the thrombosis site, maintaining the hypoxia, initiating the necrotic and apoptotic mechanisms of nerve cell death. Besides, in infants with combined impairment of central nervous system and extra-cerebral pathology the adverse course of the period of adaptation to new living conditions may become the basic cause of inadequate blood supply to cerebral structures with the decrease of the cerebrovascular reserves [13; 19; 90; 223; 230]. Certainly, all by itself, the process of adaptation of a fetus and a newborn to new conditions of existence is physiological, but the mother’s diseases occurring before and during the pregnancy increase the probability of developing both ischemia and hemorrhages into cerebral structures of newborns and infants at all pre- and post-natal adaptation stages [25; 90; 140; 163]. Additional risks include intra-uterine infections, head and neck injuries, systemic bowel diseases, autoimmune diseases, water depletion, infertility in the mother’s history, premature rupture of fetal membranes, the mother’s pre-eclampsia [22; 34; 219]. Burdened perinatal anamnesis occurs in every fourth patient and, it is associated more with neonatal and fetal stroke than with a CVD in an elder age group [34; 223].
Radiation-induced vasculopathies with subsequent vascular stenosis or occlusion more often occur in patients with brain tumors. A recent study showed that after radiation therapy courses the radiographic signs of strokes were noted approximately in 6% of children with tumors of the central nervous system [92].
One of the causes of brain blood supply disturbance in children is a cerebrovascular pathology. Presently, many authors come to the conclusion of an imperative need in a more detailed examination of children for presence of cerebrovascular diseases. In the situations, which require a compensation by means of the blood flow redistribution (e.g., during a prolonged head tilt), the decisive role may be played by specific anatomic features of major arteries of the head and the neck as well as of the basilar vessels, which can often be found even in healthy people (non-closed Willis’ circle, hypoplasias of individual artery segments or of whole arteries, stenosis / occlusion of arteries, pathological deformation of ICA) [7; 12; 30; 108].
The prevalence of ischemic disturbances, which, as a rule, are preceded by a chronic cerebrovascular insufficiency, in the structure of the young age stroke requires a comprehensive study of age peculiarities in the cerebrovascular system functioning. In principle, the brain blood supply physiology in children does not differ from that of adults. For instance, a brain weight is only 2% of the total weight of man, but it receives about 20% of the blood volume during the cardiac output and consumes 20% of oxygen. An adequate cerebral blood flow sufficient for sustaining a normal life activity of the brain is, on the average, 45—60 mL/100 g of brain tissue per minute. The proper brain activity depends on regular and adequate blood supply due to its high metabolic activity and lack of any significant energy reserves. The state of the cerebral blood flow insufficiency known as a cerebral ischemia may be either acute, or chronic and may have either local or widespread nature. When the blood flow decreases to 20—35 mL/100 g/min., the electrical activity of the brain drops, but the brain tissue changes are reversible. This state is often defined as a «penumbra» — an ischemic penumbra. When the blood flow decreases to 10—15 mL/100 g/min., the changes developing in the brain tissues become irreversible. This process leads to the death of cells (brain infarction). Timely restored adequate cerebral blood flow is enormously important in treatment of a specific group of patients with a chronic cerebral ischemia caused by a pathology of major cerebral vessels [92]. The study of cerebrovascular disturbances may turn to be a connecting link between a cerebrovascular pathology in children and the subsequent development of strokes in adults.
The anatomy of brachiocephalic arteries varies in individuals. Both in children and adults, the Willis’ circle is formed correctly only in 18—20%. Many authors think that the anatomy of cerebral vessels does not depend on gender, age or race [89; 150]. In the opinions of other authors, in children the specific anatomic and physiological features of BCA and intracranial arteries take shape, mainly, in the younger age group. For instance, the collateral vessels between ECA and ICA via a. ophtalmica are formed only in 17—22% of children [173]. The morphometric parameters of ICA grow in school-aged children from 7 to 18 years old. The morphometric parameters of common carotid arteries grow leapwise. In girls, the maximum growth occurs earlier than in boys [6].
More than a century ago in literature there were already mentions made of specific anatomical features of ICA structure — tortuosity, kink and looping. Many anatomists described bent, deformed ICA. W. Coulson (1852) was, probably, the first, who mentioned the ICA looping describing it as «a pulsating tumor on the neck». The connection between the pathologic deformations and the high risk of the CVD development was first noted by M. Riser et al. in 1951 [218]. F. McDowell et al. (1959) reported 20 cases of pathological tortuosity of ICA and related blood flow disturbances, when studying 68 patients with cerebrovascular pathology among adults over 30 years old without any proofs of occlusion found, which was supported by angiography [92]. H. Metz et al. (1961) held a retrospective survey of 1000 angiograms and found 161 cases of kinks and looping on ICA, including children below 10 years old [182]. In 1965 after having described 2,453 angiograms J. Weibel and W. Fields revealed a high occurrence of this specific feature of anatomical structure in the population — up to 10—15% cases [274]. N. Sarkari et al. (1970) first described 8 children with the pathological tortuosity of ICA combined with a chronic cerebral ischemia, of which 7 children were below 10 years old [228].
The development of contemporary diagnostic methods led to more frequent detection of ICA deformations in a population. According to US test data, the detection rate of ICA tortuosity reaches 25—30% in adults and 43% in children (R. Hobson, 2004). According to data of other authors (E. Ballota et al. (2005), G. La Barbera et al. (2006), W. Perdue et al. (1975), C. Togay-Isikay et al. (2005)), pathological deformations occur in 10—40% of cases depending on the population surveyed.
In the opinion of W. Fisher (1982), the surgical treatment of cerebrovascular insufficiency in children is required in cases, when the ICA changes may lead to a progressing neurologic deficiency. The author also states that cerebrovascular insufficiency in children presents a serious problem, as an inadequate perfusion of brain tissue leads to irreversible changes with the subsequent formation of a neurologic deficiency. In his opinion, the most frequent causes leading to cerebrovascular insufficiency are the following ICA changes: 1) congenital pathological tortuosity (kinking), 2) post-traumatic changes with thrombosis, aneurysm and embolism, and 3) thromboses [97].
In children, CVDs may be also caused by: the moya-moya disease, a fibro-muscular dysplasia, an artery dissection [8; 20]. All the above-mentioned diseases lead either to stenosis or luminal occlusion [213].
Fibro-muscular dysplasia is one of the causes of a pathological deformation of the ICA and a pediatric stroke. The pathomorphology of the fibro-muscular dysplasia is characterized by the emergence of hyperplasia sites on the connective tissue, an irregular atrophy of muscular fibers and degenerative changes of vascular walls. All this leads to formation of stenosis sites interspersed with post-stenotic aneurysms. This variant of impairment of brachiocephalic vessels may be isolated and generalized, more frequently unilateral, although a bilateral impairment is not ruled out [211].
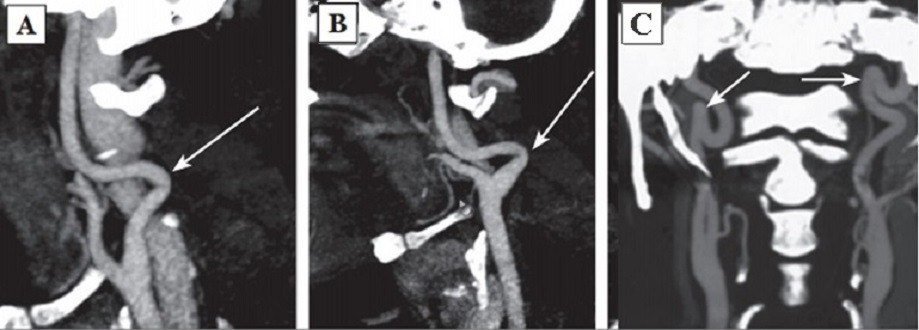
The permanent effect of the force vector of arterial blood pressure on dysplastic vascular wall was discussed earlier as the most intensive initiating agent forming a pathologic tortuosity of the ICA [145]. In 1961 H. Metz offered his own classification of ICA deformations. The author supposed that the intensity of the process depended on dysplastic changes of the vascular wall and vessel configuration and divided septal stenoses of the ICA into three types:
Type 1: artery kink at an angle over 60°(Fig. 2A);
Type 2: artery kink at an angle from 30 to 60°(Fig. 2B);
Type 3: artery kink at an angle less than 30°(Fig. 2C) [182].
In 1974 O.V. Voronin singled out three groups of pathologic deformations: looping; acute angle formed by arteries; artery tortuosity without distinct angulation. In 2001, based on 250 case follow-ups, P.O. Kaznanchyan et al. divided pathologic deformations into the following basic groups:
1. C- or S-shaped tortuosity;
2. artery elongation and artery kink at an angle of less than 90°(angulation) causing a local stenosis of a major artery;
3. pathologic looped or spiral tortuosity as well as knotting (up to 360°);
4. combination of various deformations [12].
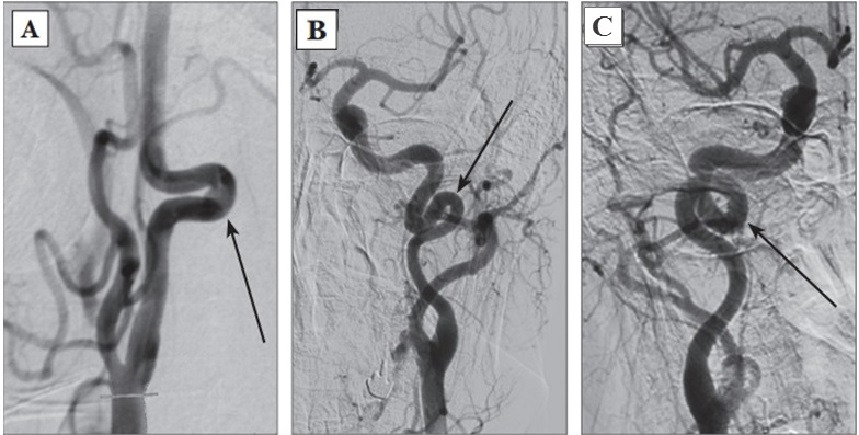
Presently, the modified J. Weibel — W. Fields and H. Metz classification is actively used:
1. Tortuosities: C- and S-shaped elongation of the ICA or deformation along the ICA course (Fig. 3A, B);
2. Insignificant deformation: angulation or kink between two ICA segments with a loop formed at an angle exceeding or equal to 60°, causing a local stenosis of a major artery (Fig. 2A);
3. Moderate deformation: angulation or kink between two ICA segments with a loop formed at an acute angle equal to 30—60°, causing a local stenosis of a major artery (Fig. 2B);
4. Marked deformation: angulation or kink between two ICA segments with a loop formed at an acute angle less than 30°, causing a local stenosis of a major artery (Fig. 2C);
5. Looping or knotting: excessively long ICA forming a marked S-shaped tortuosity or an annular configuration, where more than two ICA segments lying on different planes are involved in the process (Fig. 3C) [264].
Another important property of a deformation is its hemodynamic significance determined by the growth degree of the linear blood flow rate (LBFR) in the deformation area due to its local contraction, thus reflecting the intensity of septal stenoses and twist of the artery without distal hypoplasia signs and decrease of volumetric blood flow in the deformed artery. The tortuosity is considered hemodynamically significant, when the increasing LBFR in the deformation area exceeds 170 cm/s (moderate significance). If the LBFR exceeds 250 cm/s, it is considered as evident hemodynamic significance, and when it exceeds 300—350 cm/s with the presence of a turbulent noise — as rough [26].
According to literature data, a forward course of major vessels is noted in 65—70% of persons, vascular deformations — in 23—40%, including the pathological, hemodynamically significant tortuosities — about 9—16% of cases. According to data of J. Weibel and W. Fields (1965), C- or S-shaped course of a vessel elongated approximately by 4 cm occurs twice more often unilaterally than bilaterally. Other types of pathological deformations are revealed equally often, regardless of the gender and the age, while unilateral pathological deformations occur also twice more often than bilateral [14; 20]. Hemodynamically significant, pathological tortuosities cause both acute cerebrovascular diseases and chronic cerebrovascular insufficiency. While a child grows, the pathological tortuosity of the ICA may be leveled completely or the artery may get «straightened», which is accompanied by a recovery or an improvement of the blood flow and the regression of neurologic disturbances [14].
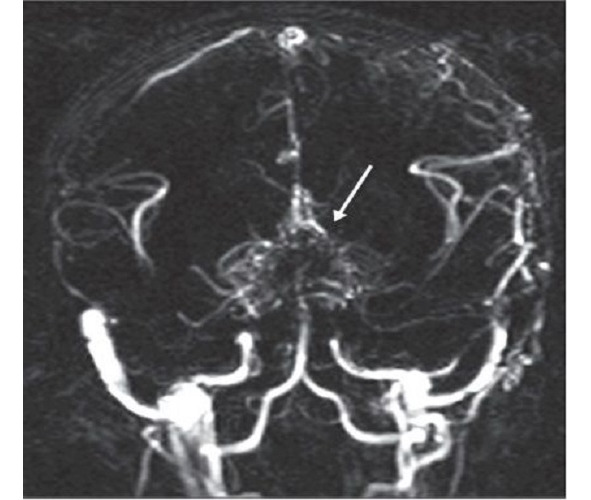
Moyamoya is a rare disease [208] characterized by a progressive spontaneous stenosis or occlusion of a supraclinoid segment of an ICA (single or both) at the level of the siphon and the initial segments of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA) with the subsequent involvement of the VBS. The specific feature of this disease is the secondary formation of a basilar, anastomotic capillary network, resembling a small cloud of smoke (Fig. 4) during the angiographic imaging, which is pronounced in Japanese as «moyamoya». This word has become an official name of the disease. In 40% of cases in the moyamoya disease a bilateral impairment of the ICA is noted; initially the ICA is involved only on one side [233; 285]. The «moyamoya syndrome» term is more often used for angiographic description of the pathology [266].
According to data from various sources, the «moyamoya» term was first used by A. Takeuchi and J. Suzuki in 1969 [254]. According to other data, the discovery of this disease is related to an earlier period, when in 1937 K. Shimizu implemented the carotid angiography technique in Japan [205]. Shortly after the World War II, neurosurgeons started to apply this type of test actively, which permitted to diagnose and to study the moyamoya disease. It was found then that this pathology can be often seen in the countries of South-East Asia. The research works by A. Takeuchi, K. Shimizu (1957), N. Moriyasu (1964), T. Kudo (1968), J. Suzuki and A. Takaku (1969) have contributed a lot into the worldwide awareness and study of the disease.
It was shown that the highest morbidity rate of the moyamoya disease in the world is noted in Japan — 4—5 incidents per 100,000 population annually. By way of contrast, in 2005 in America the morbidity rate of the moyamoya disease was 0.086 incidents per 100,000 population [266]. R. Smith and J. Scott (2012) claim that in America the moyamoya disease rarely occurs in children — 1 incident per 1,000,000 — and becomes the cause of 6% of all pediatric strokes [243]. According to data of various European clinics, for the last 5—6 years the number of patients, especially children, with the moyamoya disease has increased in Europe, and it still continues growing [139]. Such a tendency might be connected with the improved diagnostics of cerebrovascular diseases, although this issue is studied little. Some prevalence of the morbidity is noticed in women (the ratio of female and male patients is 1.6:1). I. Ahn et al. (Korea, 2014) analyzed the statistical data for the period from 2007 till 2011. In 2011 the total number of patients with the moyamoya disease was 8,154 in Korea, during the period from 2007 till 2011 the morbidity was recorded on the level of 2.3 per 100,000 people, while in 2011 it was 16.1 per 100,000 people; the ratio of female and male patients was 1.8:1 [41].
In Russia there are singular publications about this disease [15; 18; 29].
The moyamoya disease has two age-related peaks of clinical manifestation: the first one comes on children at the age of 5—10 years old, while the second one — on the age of 30—40 years old [258; 266]. The pathologic process is most active approximately until the age of 10 years, and it gets stabilized approximately by the age of 20 years [208; 270].
The etiology of this disease is actively discussed, although it still remains unknown [208]. It is supposed that the moyamoya disease may be either congenital or acquired [133]. It is noticed to be associated with other systemic and non-systemic diseases (Down’s syndrome, neurofibromatosis type I, autoimmune diseases, tuberous sclerosis, atherosclerosis, fibro-muscular dysplasia, thalassemia and sickle-cell anemia, thyroid disorders) as well as with radiation therapy of basilar gliomas in children with a craniocerebral injury [135; 175; 224; 234; 270].
Hereditary factors play an important part in the moyamoya disease [196; 277]. There are some known familial cases of the disease. According to some data, this disease has a familial nature approximately in 15% of patients. Due to this, over the latest years the attempts have been made to find a genetic base of the disease. Some data were published on the detection of the locuses associated with the moyamoya disease on 3, 6, 8 and 17 chromosomes. In 2008 some data were published about the autosomal dominant inheritance (the gene was mapped to chromosome 17q25) of the moyamoya disease [119; 130; 197; 199; 225]. However, in the literature there is a description of the moyamoya disease in one of two twins, and this permitted the authors to conclude that this disease was not rigidly determined [256]. In one of their latest works J. Ma et al. report (China, 2013) that while studying the associative genetic predisposition, they have found the connection between the moyamoya disease and genic polymorphisms in RNF213 gene (p.R4859K and p.R4810K), which is more common in Japan and Korea, but less common in China [171].
Despite these studies, the genetic screening in the moyamoya disease has not become widely common, because its efficacy was not proved [233]. As of today, the researchers studying this problem think that this disease has a multi-factor nature [266].
As already stated above, the pathophysiology of the moyamoya disease consists in the gradual constriction of major stems of basilar intracranial arteries due to deposits of lipids in the intima in the absence of inflammation signs. The middle layer of arteries is thinned, the adventitia is not involved into the process. Similar changes in vessels may be observed in other organs, which shows that the vascular impairment has a systemic nature. The involvement of the immune system into the process is not ruled out [270]. In some authors’ opinion, inflammatory proteins participate in the development of the disease [287]. Anyway, intra- and extra-cranial vascular anastomoses are formed on the base of the brain during the progressive occlusion process in the vessels of the Willis’ circle [270; 277], which, to a certain degree, compensates for critical decrease of the regional cerebral blood flow, but leads to a gradual growth of chronic cerebral ischemia mostly in the cortical sections of big hemispheres [208; 266; 291]. The anastomotic capillary network is disappearing, while collaterals are developing from the ECA (meningeal collaterals are called «a magic network») [208; 291]. Arterial aneurysms frequently occur in the moyamoya disease. According to some data, the detection rate of VBS aneurysms reaches 62%, which several times exceeds the incidence of this pathology in the population (5—15%) [196; 270].
The unique character of clinical manifestations of the moyamoya disease and syndrome consists in the fact that this pathology may reveal both as an ischemic CVD and intracranial hemorrhages. Also, both these variants may occur in the same patient during his life [192; 196; 270]. In 1990 Y. Matsushima offered the moyamoya disease classification based on its clinical course:
Type 1: revealing as rare TIAs — 2 times per month or more seldom;
Type 2: revealing as frequent TIAs — 2 times per month or more often;
Type 3: revealing as a minor stroke (with regressive neurologic deficiency within 2—3 weeks). Small ischemic lesions may be found on a brain CT;
Type 4: revealing as a progressive stroke (gradual growth of neurologic deficiency with time);
Type 5: revealing as a complete stroke resulting in the formation of a persistent neurologic deficiency; extensive ischemic lesions are found on the cerebral tissue during the brain CT and MRI.
Types 1—5 are referred to ischemic variant of the disease course.
Type 6: the disease reveals as a hemorrhagic CVD due to a rupture of anastomotic network vessels [178].
C. Mohanty et al. (India, 2013) report 11 incidents of an unusual course of the moyamoya disease, when both hemorrhagic and ischemic CVD lesions are noted in the same hemisphere of a patient either simultaneously or at different periods of time [186].
According to foreign data, the moyamoya disease mortality is higher in adults than in children (10 and 4.3% respectively). Hemorrhages were the cause of death in 56% of 9 perished children. With the surgical treatment a favorable prognosis is noted in 58% of cases [268].
4. Clinical manifestations of cerebrovascular diseases in children
Clinical manifestations of acute cerebrovascular diseases in the carotid system of children are typical enough, and they reveal in a focal neurologic deficiency with developing motor disturbances (87—95%), disturbances of speech, sensibility and vision as well as other symptoms corresponding to the location of cerebral tissue lesions. In massive strokes during the acute period of the disease, as a rule, general neurological symptoms are more marked, which is caused by edema and dislocation of the brain. When the ischemic zone is small, the focal symptoms develop in the background of the generally normal state. The clinical picture of a cerebrovascular disease in a child may be atypical: nausea, vomit and depression of consciousness may be replaced with agitation or body temperature rise and convulsions (19—58%).
For obtaining more objective data on the severity of clinical manifestations of the ACVD and assessing the neurologic deficiency changes in acute and rehabilitation periods of the stroke, the PedNIHSS (Pediatric National Institute of Health Stroke Scale) scale is used, which has showed its consistence with the NIHSS scale [175; 235]. During the period of residual effects, American researchers apply the PSOM scale (Pediatric Stroke Outcome Measure) [37; 107]. These scales are rather extensive, but easy to fill in, so they can be automated and introduced into the patient examination standard. The obstacle for their implementation in the RF territory is the need for training of specialists in the estimation methodology proper as well as the need for validation of evaluation and measurement scales.
A paroxysmal syndrome often becomes the first symptom of any type of a CVD in children. More frequent registration of the paroxysmal syndrome is noted in the infant group [60; 69; 81; 291]. In children, the paroxysmal syndrome is typical not only for a stroke, but also for various brain damages (mass lesions, etc.). Due to its high diagnostic significance, it is recommended by the American Epilepsy Society as a mandatory indication for brain MRI [122].
The emergence of convulsions at the onset of a cerebrovascular pathology is a relatively unfavorable sign. It is proved that it is specifically the ACVD manifestation in paroxysms recurring soon, that is associated with the unfavorable prognosis regarding the patient rehabilitation and the restoration of the focal neurologic deficiency as well as with the risk of emergence and severe course of symptomatic epilepsy [99; 125; 259]. Normally, convulsions are not the only manifestation of an ACVD. A focal neurologic deficiency develops either alongside with them, or in the later periods [35; 220; 280]. The largest samplings of patients with an ACVD and formation of a post-stroke epilepsy during the subsequent period of the disease are presented in Table 1.
Table 1: Epilepsy formation risk in children, who had suffered an ischemic stroke (according to literature data from 2010 till 2015)
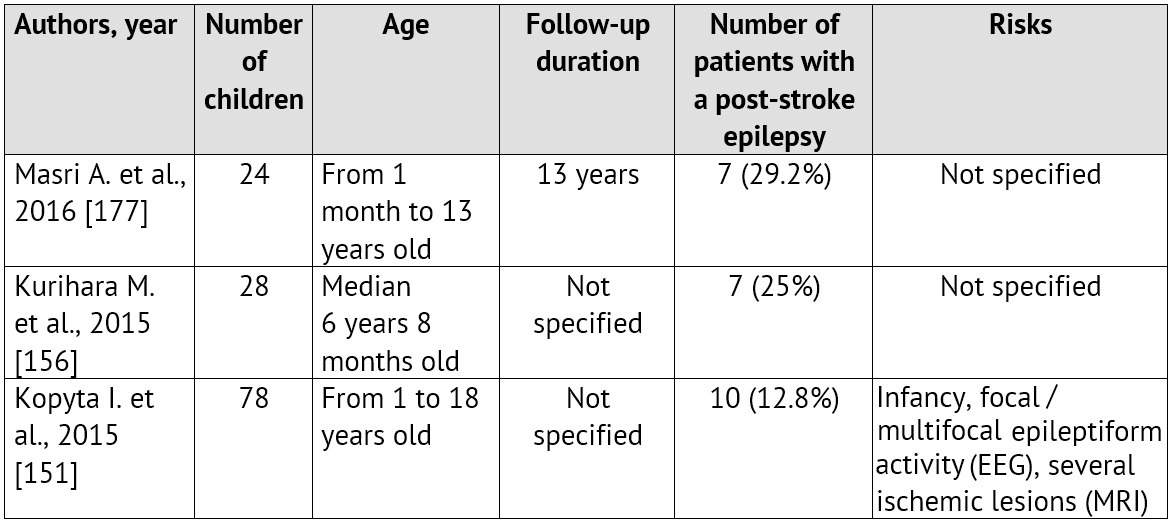
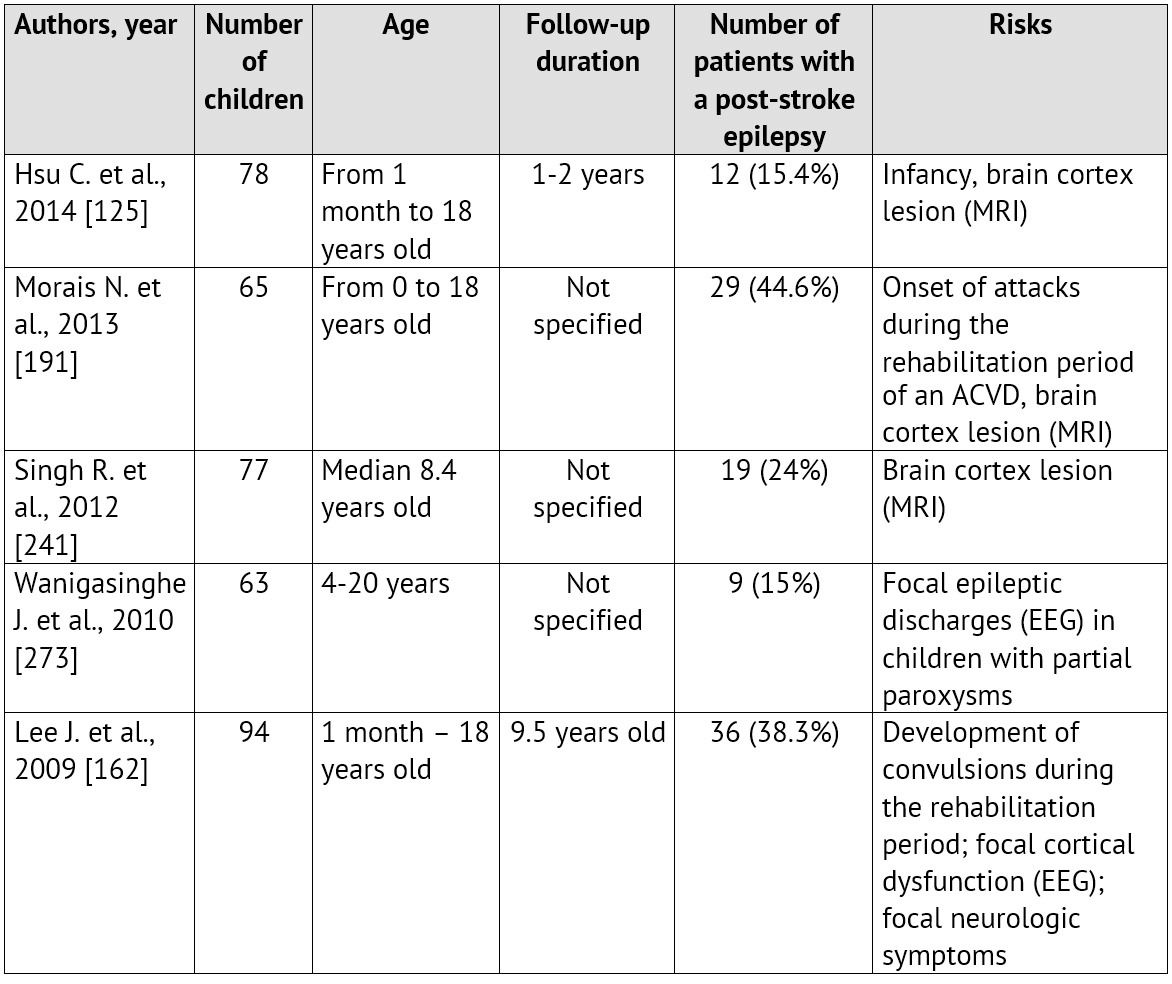
On the other hand, the first manifestations of the disease may be rather non-specific: an isolated degradation of consciousness level or a headache, which, considering age, difficulties in awareness and in verbalization of unusual symptoms by the child itself as well as lack of «stroke» alertness in pediatric neurologists, leads to an essential delay in neuroimaging and ACVD diagnosing [24; 60; 63; 67; 179]. These circumstances often lead to a rather paradoxical situation called «clinicodiagnostic scissors»: children with CVD symptoms are hospitalized promptly enough, but they do not get adequate verification of the diagnosis, including an instrumental one, and, therefore, they do not get the treatment.
Pediatric patients find themselves in a hospital, on the average, within the first three hours from the onset of the ACVD symptoms, but they get to the neuroradiologists within 8 hours, whereas for adult patients these deadlines are 8 and 2 hours respectively [63; 103; 179; 247]. Transient motor and/or sensorial disturbances in the structure of partial attacks lead to the initial diagnoses, which are most common in children (epilepsy, neuroinfection, crainio-cerebral injury, etc.) and which, therefore, occupy top positions in the immediate memory of emergency phase doctors [103; 247].
During the analysis of the clinical picture in children with IS, examined by specialists of the FSBEI of Higher Professional Education «Urals State Medical University», the following data on the varying occurrence of clinical symptoms were obtained in 162 children (Table 4). Within the first 24 hours of the disease the comparability was noted between the registration rates of general cerebral and focal neurological symptoms. During the acute period of the IS, the most typical combination of symptoms in children were the degradation of consciousness level and the central pareses of limbs and mimic muscles.
Practically, every fourth child at an age enabling the adequate assessment of these symptoms had signs of ataxia and speech disturbances. Thus, the most prominent combinations of symptoms, which form the diagnostic rules such as «Give me five» and FAST in stroke diagnostics at the age typical for IS and TIA, can also be successfully applied in pediatric practice.
The spectrum of focal neurologic symptoms in patients was consistent with the blood supply systems and the infarction location. In the hospital stay period, the neurologic deficiency persistence had a direct positive connection with the size of the stroke zone on CT or MRI (r=0.56, p <0.05).
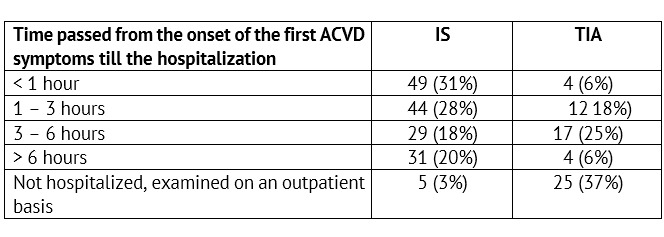
The rate of admitting children with verified IS (n=158) and TIA (n=62) to the specialized care delivery stage was analyzed (Table 2).
Thus, only a little more than half of the children with IS get to the specialized care delivery stage within the reported deadlines accepted for hospitalization of adults — within the boundaries of the so called «time slot» (up to three hours) — 59% (n=93), when thrombolysis is possible. For children with TIA the onset of focal or general cerebral symptoms did not remain unnoticed also in more than a half of cases — 60% (n=37). On the other hand, a short-term and transient nature of symptoms in TIA have led to the lack of the emergency hospitalization and to scheduled health-seeking in 40% of children in this group (n=25).
Blood circulation diseases of the VBS have such typical symptoms as vertigo with nausea and vomit, disorder of static equilibrium and gait, ataxia in limbs and nystagmus, and such less typical symptoms as pareses in the limbs and various sensibility variations, lesions of cerebral nerves (CN) caused by a cerebrovascular disease in the brain stem. In ischemia of occipital lobes there may be some disturbances of visual functions [17].
Table 2: Time passed from the onset of the first ACVD symptoms till the admission to an emergency / neurology specialized healthcare facility
A chronic cerebrovascular ischemia most often manifests itself in signs of a dyscirculatory encephalopathy (DEP) characterized by diffuse-type headaches, vertigo, tinnitus, memory derangements, emotional lability, increased fatigability and performance impairment, sleep disorder. In DEP, general symptoms prevail without focal neurologic symptoms: severe headaches (77%), increased fatigability (68%), hypomnesia (44%) [17]. Decreased learning capacity is typical for such children.
Regarding the specific features of the clinical course of some types of the pathology, it should be mentioned that clinical manifestations of stenosis and occlusion of major cerebral vessels do not have any specific features and can manifest themselves as various ischemic cerebrovascular diseases. A moderate decrease of the cerebral blood flow usually does not manifest itself clinically (asymptomatic disease course), or it may be accompanied by some unspecific complaints.
At the onset of the moyamoya disease and syndrome, their clinical manifestations are rather diverse, and they may resemble the clinical manifestations of cerebrovascular disturbances in pathologic deformations of major cerebral vessels, thrombosis and atherosclerosis of intracranial arteries as well as the manifestations of other diseases (epilepsy, malformations of cerebral vessels, subarachnoid and intra-cerebral hemorrhages of various genesis [192; 196; 270].
Headache is mentioned as a manifesting symptom in a variety of studies on the moyamoya disease occurring in children too. In literature, there is even an individual notion — a headache associated with the moyamoya disease (HAMD) [141; 233; 238; 288]. Headache is often the only symptom at the onset of this disease. The headache is supposed to be caused by a compensatory dilatation of meningeal and leptomeningeal arteries, which can stimulate nociceptive receptors of dura mater of brain (DMB). The headache may have a migraine-like nature and be resistant to a drug therapy. However, this symptom usually is not considered as a fatal sign. In most patients, the headache regresses after the surgery [71; 136; 238].
The subsequent joining of transient focal neurologic symptoms is often considered by neurologists as a manifestation of sub- or de-compensation of residual organic background under the effect of school loads, intense sports activities, viral infections, vaccinations, etc. They are acknowledged as TIAs most often retrospectively, after the verification of the moyamoya disease. A short-term and transient nature of symptoms in children, often combined with the inability to describe their «unusual» complaints verbally, result in delayed help-seeking and late hospitalization. According to literature data, the delayed diagnosing is noted in all patients, and it may exceed two years. The moyamoya disease is usually identified only after the child has suffered a typical IS, which is followed by various neuroimaging examinations. Clinical manifestations in children with the moyamoya disease are distributed in the occurrence rate as follows: ischemic symptoms — 80% of cases (including strokes — 40% and transitory ischemic attacks — 41%) [21; 80; 224; 242; 254]; epilepsy — 5%, intracranial hemorrhages — 2.5%; other symptoms — 12.5% of cases (headache, motor disturbances, or combined symptoms) [72; 80; 224; 242; 254].
5. Diagnostics of pediatric stroke
A nation-wide Russian recommended list of diagnostic procedures aimed at differential diagnostics of a CVD at an early age does not exist. It is actively discussed and formed in some individual centers. The variability of the CVD causes considerably hinders the diagnostic search. During the most acute and acute disease periods, the efforts are focused on identifying the pathogenic variant of the CVD, primarily, on identification of the most frequent diseases, whose therapy can be started immediately (cardiac pathology, congenital clotting disorders, congenital and acquired pathology of cerebral vessels) with due regard for the age [22; 34]. If the cause has not been determined, it is recommended afterwards to rule out consecutively other, less common, causes of the CVD at an early age [7; 9; 22; 24; 134].
It is known that, despite a rather well-defined organization of the diagnostic process in foreign clinics, about 20% of ischemic strokes remain etiologically unexplained. The similar national indicator reaches 65 — 70% [161; 174; 280]. Despite the high cost of a laboratory-based instrumental examination and its long duration, such an examination must be held. The earliest clarification of the cause of an ACVD in a child is considered a high-priority and the most important mission of a diagnostic search at any phase of the disease. The accurate clarification of the etiology of an ischemic ACVD determines the focus of the corrective drug therapy, the system of preventive measures and the prognosis regarding the further course and recurrence of the disease.
Based on the FSBEI of Higher Professional Education «Urals State Medical University», a list of diagnostic activities, which are due to be held for examination of children with IS and TIA at an emergency (inpatient) stage, was developed and implemented into a routine practice (Table 3).
It must be noted that a consecutive clinical, laboratory-based and instrumental examination with the fulfillment of all items proposed above should be held even in the cases, when the cause of a stroke or TIA seems evident. This is due to the fact that, beside an evident cause, the presence of other, equally important, risks may be possible, which could lead to a pathology of the blood coagulation system. It is just these individual risk combinations, which, under the effect of the initiating agents, facilitate the realization of genetically determined prothrombotic readiness and lead to the formation of a focal spot of an infarction or TIA in children.
All stated above is aimed at the clarification of the ACVD causes as well as at the further prevention of recurring strokes. However, regarding the determination of indications for surgical treatment held, when the conservative therapy is not effective, or in addition to it, as well as when a surgical intervention method is selected, some additional tests are required to assess the state of major vessels and the cerebral circulation system. As distinct from adults, the algorithm of instrumental examination of children with cerebrovascular diseases is not developed yet.
During several decades, a traditional direct, cerebral, selective angiography (CAG) developed by E. Moniz in 1927 was used for verification of a cerebrovascular pathology [188; 189; 227]. Lately, the direct angiographic examination was practically ousted by such methods as spiral computed angiography (SCT AG) and magnetic resonance angiography (MRA). Nevertheless, the CAG remains indispensable in the evaluation of the collateral blood circulation.
Table 3: Scope of diagnostic activities during the initial hospitalization of children with ischemic ACVD
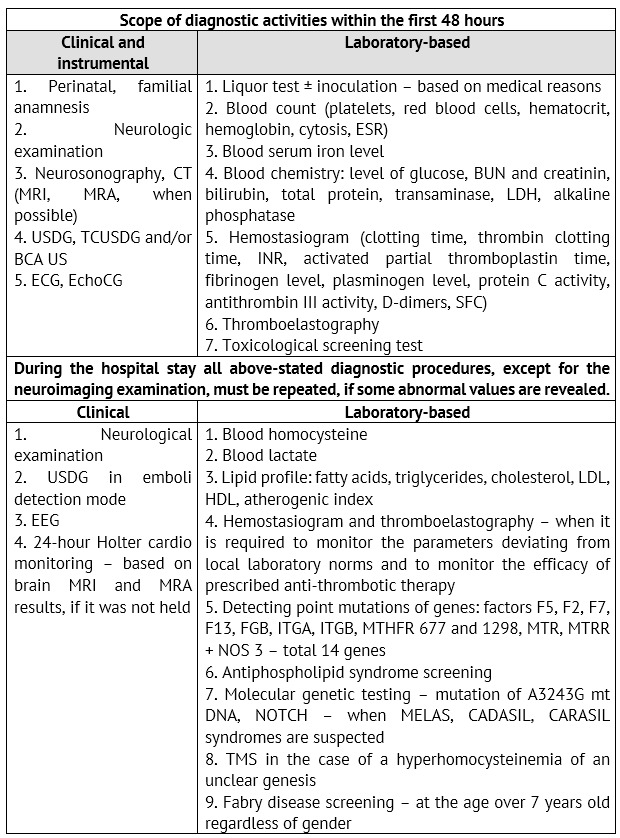
The emergence of ultrasonic test methods became an important stage in the cerebrovascular pathology study. These methods have become quite common due to their non-invasiveness and high information capacity. Presently, such methods are used as ultrasonography of brachiocephalic arteries (BCA US) with colored mapping, transcranial ultrasound dopplerography of cerebral vessels (TCUSDG).
The methodologies, which permit to evaluate the brain perfusion and its reserves, include: SCT perfusion, MR perfusion, positron emission tomography (PET).
Over many decades, brain functionality has been assessed by means of the electroencephalography (EEG). The study of cerebrovascular reactivity by applying the loading tests with hyperventilation during the EEG (curve response delay and clinical manifestations) and the neuropsychological examination permit to clarify the indications for surgery, the side status of the lesion as well as to follow up the brain state changes after the surgery.
In each specific situation, the choice of instrumental examination methods was determined by the volume of data required for identifying the indications for surgical treatment as well as for planning a surgical intervention.
During the additional examination of children the basic tasks aimed at selecting the approach to surgical treatment are:
1. Evaluating the state of extra- and intra-cranial cerebral arteries and identifying the pathology type (stenosis, occlusion, pathological tortuosity);
2. Evaluating the hemodynamic significance of revealed changes and identifying their effect on the cerebral hemodynamics;
3. Evaluating the compensation abilities of collateral blood circulation: specific anatomical features of the Willis’ circle, assessment of its functional state at rest and in loading tests, presence of natural extra-intra-cranial anastomoses, blood flow symmetry.
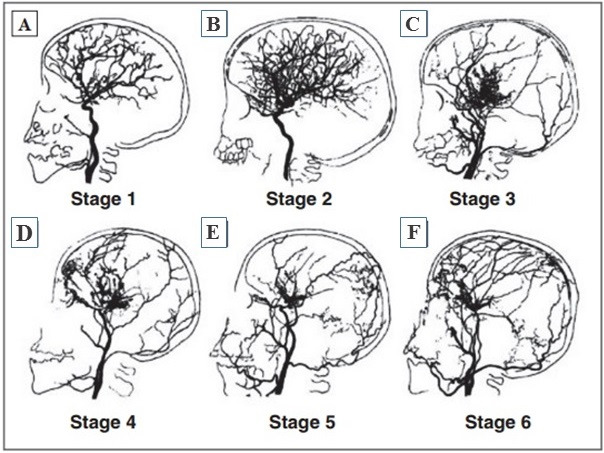
When the cerebrovascular system state is evaluated in patients with the moyamoya disease, the angiographic classification proposed by J. Suzuki in 1969 is relevant (Fig. 5). It permits to determine the phases of the obliteration process in the vessels and the status of the vascular system as of the examination moment:
1. Stenosis of the supraclinoid portion of the ICA, usually bilateral (Fig. 5 A);
2. Development of vascular anastomoses on the base of the brain (Fig. 5 B);
3. Growth of ICA stenosis and intensity of the anastomotic capillary network on the base of the brain (in most cases the disease is diagnosed at this phase) (Fig. 5 C);
4. Occlusion of the whole Willis’ circle and PCA, the starting point of the onset of extra-cranial anastomoses (Fig. 5 D);
5. Reduction of the anastomotic capillary network (Fig. 5 E);
6. Complete disappearance of moyamoya-type vessels and main cerebral arteries (Fig. 5 F) [254].
Apart from the CAG, in the moyamoya disease there are other useful examinations: EEG, TCUSDG, SCT-perfusion, MRI-perfusion, PET.
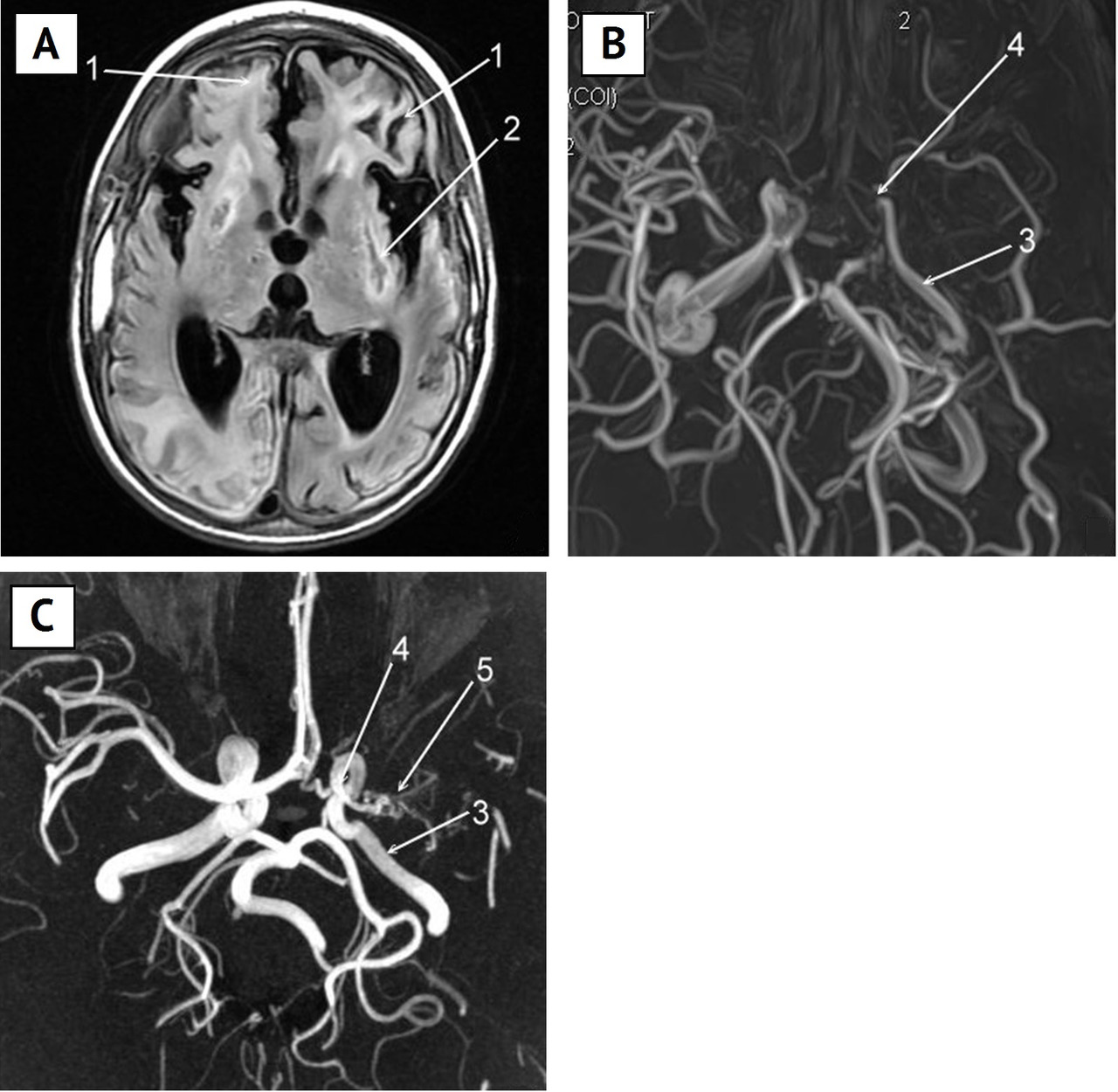
Presently, in foreign literature on patients with the moyamoya disease, a great importance is attributed to the presence of a cortical ischemia and detection of an “ivy sign” on the brain MRI image. The “ivy sign” is characterized by an increased leptomeningeal signal in FLAIR mode (fluid-attenuated inversion recovery) along the cortical surface of cerebral fissures and DMB (Fig. 6 A). Some authors [192; 205] supposed that this symptom points at the insufficiency of the cerebrovascular reserve.
The latest studies showed [193] that the “ivy sign” is more intensive in the hemisphere on the side of worse imaging of the MCA in angiography. A correlation was revealed between the signal intensity and the clinical course of the disease: the more intensive it is, the more severe is the manifestation of the chronic cerebral ischemia. After the surgical treatment (brain revascularization) this signal becomes less intensive [193].
Chapter II.
Conservative therapy of stroke and approaches to secondary prevention of CVD
1. Conservative therapy in the acute period of stroke
So far, for pediatric patients with CVDs there are no developed or approved guidelines on medical aid at all stages of inpatient and outpatient treatment. According to the recommendations of the American Heart Association & American Stroke Association (2012), during the acute period, it is necessary to use the general principles of life activity control, to maintain the general volume of circulating blood, to stabilize the hemodynamics (avoiding hypotension), to maintain the normal body temperature (avoiding hypothermia / hyperthermia), regardless of the CVD type (Class 1, Level of Evidence A) [107; 280].
Presently, the top priority in the treatment of ischemic-type ACVDs in adults is attributed to a thrombolysis. The whole system of measures related to emergency care implies the determination of indications and the evaluation of the technical feasibility of thrombolysis. The literature presents successful attempts of thrombolytic therapy at the most acute phase of the IS and in childhood [91; 117; 272; 260]. Despite the high efficacy (up to 80%), the international pediatric community laid a moratorium on the application of all thrombolysis methodologies due to a high risk of complications. It was proposed to maintain the moratorium until some new data on the efficacy and safety of this therapy are published (Class III, Level of Evidence C) [45; 48; 201; 280]. An exception is provided for clinical studies in a teenagers’ group — the thrombolysis may be applied, if a teenager meets the inclusion criteria [44; 45; 58; 280].
Regarding the application of anticoagulants and disaggregants, the high level of evidence has not been reached yet. The researchers agree that a low molecular weight heparin may be recommended for all patients with ischemic CVDs within one week, until the cause of the stroke is determined (Class II, Level of Evidence C), as well as in some variants, whose etiology and pathogenesis are known [65; 85; 280].
The list of other methods is rather limited, and it includes control of hydration, hypoxemia and hypotension during an acute period of the disease, regardless of the CVD type, and regular blood transfusions in children with a sickle-cell anemia.
Бесплатный фрагмент закончился.
Купите книгу, чтобы продолжить чтение.