
Бесплатный фрагмент - Herpetic keratitis: from symptoms to recovery
Herpetic Keratitis: From Symptoms to Recovery
There are contraindications. Consultation with a specialist is necessary.
I would like to express my deepest gratitude to my beloved wife, Anna, whose unwavering help, steadfast support, and boundless patience have been the cornerstone of this book’s completion. Her encouragement and understanding during this demanding journey have been invaluable.
To my son, Nikita, I extend my sincere thanks for his invaluable assistance in editing the tables and diagrams, as well as his thoughtful advice on design matters, which greatly enhanced the clarity and presentation of the material.
I am profoundly thankful to my mother, Nadezhda, whose love and steadfast support provided me with the emotional strength to persevere throughout the writing process.
This book, “Herpetic Keratitis: From Symptoms to Recovery,” would not have been realized without the love, assistance, and encouragement of my family. Their contributions have been deeply cherished and have enriched this work immeasurably.
All rights reserved. No part of this book may be produced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or any information storage and retrieval system, without permission in writing from the publishers.

Introduction: The Significance of the Problem of Herpetic Keratitis
Herpetic keratitis (Herpes Simplex Virus keratitis) remains one of the most significant causes of infectious blindness worldwide despite advances in modern medicine. Its recurrent nature, complex diagnosis, and need for prolonged treatment make this disease an important topic for ophthalmologists.
Herpetic Keratitis as a Leading Cause of Infectious Blindness
Herpetic keratitis (HK) holds a leading position among infectious causes of vision loss, surpassing bacterial, fungal, and parasitic keratitis. Mechanisms of blindness include:
• Damage to the corneal epithelium and stroma with formation of irreversible scarring.
• Chronic inflammation leading to corneal neovascularization.
• Development of secondary complications such as glaucoma or dry eye syndrome.
1. Statistics:
• According to the World Health Organization (WHO), herpetic keratitis affects more than 1.5 million people globally each year, with approximately 40,000 losing vision in one or both eyes. See Appendix (QR code for Source No. 1)
• In 25% of cases, this disease leads to keratoplasty.
2. Epidemiology: Global Prevalence and Risk Groups
The global prevalence of herpetic keratitis varies depending on the region, healthcare level, and climatic conditions. General indicators:
• HSV-1 infection prevalence among the population reaches up to 90% in some regions, creating a high likelihood of ophthalmic manifestation of the disease.
• Recurrence frequency: more than 25% of patients who experienced the first episode of HK suffer recurrences within the first year, and 50% within 5 years. See Appendix (QR code for Source No. 2)
Risk groups:
• Patients with immunodeficiency conditions (e.g., HIV infection, long-term immunosuppressive therapy, organ transplantation).
— Elderly individuals with reduced regenerative capabilities of corneal tissues.
• Children and young people with a high risk of primary infection.
• Residents of regions with high levels of ultraviolet radiation, as UV exposure activates the virus. See Appendix (QR code for Source No. 3)
Climatic factor:
• Tropical and subtropical countries show a higher frequency of cases associated with viral activation by sunlight.
3. Economic and Social Burden of the Disease
Direct Medical Costs:
Treatment of herpetic keratitis requires substantial resources including:
• Prolonged therapy with antiviral drugs and immunomodulators.
• Repeated physician visits for monitoring.
• Possible surgical intervention (e.g., keratoplasty).
Recent studies indicate the annual direct treatment cost for herpetic keratitis in the United States is approximately $17.7 million, substantially lower than previous estimates. This figure refers specifically to herpetic keratitis and does not include the broader spectrum of herpetic eye disease. See Appendix (QR code for Source No.4)
Indirect Costs:
• Loss of patients’ working capacity, especially during recurrences.
• Work absenteeism and reduced productivity due to the need for regular treatment.
• Social costs including psychological stress related to the chronic nature of the disease.
Quality of Life:
Vision impairment and frequent exacerbations of HK considerably reduce patients’ quality of life, including:
• Limitation of daily activities.
• Fear of vision loss due to recurrences.
•Depression and anxiety disorders associated with prolonged treatment.
Economic burden of herpes:
According to WHO, 2024:
• Annual economic losses from herpes are estimated at $35 billion.
• Genital herpes causes significant healthcare expenses and reduced labor productivity. See Appendix (QR code for Source No. 5)
Conclusion:
Herpetic keratitis is not only a medical but also a socio-economic problem that requires an interdisciplinary approach to treatment and prevention. Its global significance dictates the need to develop new diagnostic, therapeutic, and preventive methods that will reduce disease prevalence and its severe consequences.
Brief Overview of Herpes Simplex Virus (HSV)
Human herpesvirus (Herpes Simplex Virus, HSV) is a widespread DNA-containing virus belonging to the Herpesviridae family. Its unique feature is the ability of latent persistence in the body and reactivation under the influence of external and internal factors. In ophthalmology, two types of the virus play a key role: HSV-1 and HSV-2.
1. Types HSV-1 and HSV-2
HSV-1 and HSV-2 share a similar genetic structure but differ in preferred transmission routes, tissue tropism, and disease spectrum.
HSV-1 (Herpes Simplex Virus Type 1):
Main clinical manifestations:
• Leading cause of ophthalmic herpes: causes epithelial, stromal, and endothelial keratitis, keratouveitis, and recurrent eyelid lesions (herpetic blepharitis).
• Also associated with oral herpes and encephalitis.
Transmission routes:
• Contact: through saliva, skin, mucous membranes.
• Possible transfer to the cornea through contaminated hands or objects (e.g., contact lenses).
Epidemiological significance:
• Over 60% of the adult population worldwide is infected with HSV-1.
• Predominates in countries with temperate and cold climates.
HSV-2 (Herpes Simplex Virus Type 2):
Main clinical manifestations:
• More commonly causes genital herpes but can affect the eyes (e.g., keratoconjunctivitis) in neonatal herpes.
• Less frequently involved in ophthalmic pathology in adults.
Transmission routes:
• Sexual: main mode among adults.
• Vertical: from mother to newborn during childbirth.
Epidemiological significance:
• Occurs in 10–20% of the population depending on the region, more frequent in countries with high HIV prevalence. See Appendix (QR code for Source No. 6)
Comparison of Types:
HSV-1 is more tropic to the trigeminal nerve ganglia and ocular region, whereas HSV-2 mainly affects sacral ganglia. However, both types can affect the eyes and other organs.
2. Mechanisms of Infectivity and Viral Persistence
One of the key features of HSV is its ability for lifelong persistence and periodic reactivation. These properties are explained by unique mechanisms of interaction between the virus and host cells as well as the immune system.
Stages of the Infectious Cycle:
1. Entry into the Epithelium:
— HSV binds to receptors on the surface of epithelial cells, including heparan sulfate proteoglycans (HSPG) and nectin-1 protein.
— The viral capsid is delivered into the nucleus of the cell, where viral DNA is released.
2. Replication and Assembly of Viral Particles:
— The viral genome replicates using host cell enzymes.
— Early and late viral proteins necessary for the assembly of new virions are synthesized.
3. Cytotoxic Effect:
— Lysis of the host cell leads to inflammation and localized tissue destruction, for example, of the corneal epithelium.
Mechanisms of Persistence:
Following primary infection, the virus migrates via sensory nerves to neuronal ganglia, where it can persist in a latent form:
• In the latent phase:
— Viral DNA is maintained in the nucleus of neurons as an episome (circular form, not integrated into host DNA).
— Viral protein synthesis is minimal, rendering the virus “invisible” to the immune system.
• Upon Reactivation:
— Trigger factors (such as ultraviolet radiation, stress, immunosuppression) induce active viral replication and migration back along the nerve pathways to the original site of infection.
3. Zone of Viral Latency in the Trigeminal Nerve
The trigeminal nerve (n. trigeminus) is the key structure where HSV-1 persists in latent form.
• Location: The Gasserian ganglion (ganglion trigeminale) serves as the principal viral reservoir, where the virus resides in a dormant state between recurrences.
• Mechanism of Latency:
— Viral genetic activity remains minimal due to epigenetic control.
— Latency Associated Transcripts (LAT-RNA), which are non-coding RNAs, play a crucial role in suppressing viral replication and maintaining neuronal viability.
• Viral Activation:
— Under the influence of triggers, epigenetic control diminishes, allowing viral replication and migration back to the cornea.
— Repeated activations lead to chronic inflammation and irreversible corneal damage.
4. Modern Aspects of HSV Study
Contemporary research focuses on the latent phase of HSV, prevention of reactivation, and reduction of inflammation-induced damage:
• Molecular Inhibitors:
— New drugs targeted at inhibiting specific viral proteins involved in activation are under development.
• Genome Editing Technologies:
— Use of CRISPR/Cas9 to disrupt the viral genome during latency is being explored.
• Immune Approaches:
— Vaccines capable of eliciting durable cellular immunity against HSV are being investigated.
Conclusion
HSV is a unique pathogen that combines high infectivity, latent persistence, and a recurrent nature.
For ophthalmologists, understanding the mechanisms of infection, latency, and reactivation is essential to selecting optimal strategies for treatment and prevention of herpetic keratitis.
The features of the mechanism of action of herpes simplex virus in the human body are illustrated in Scheme 1.
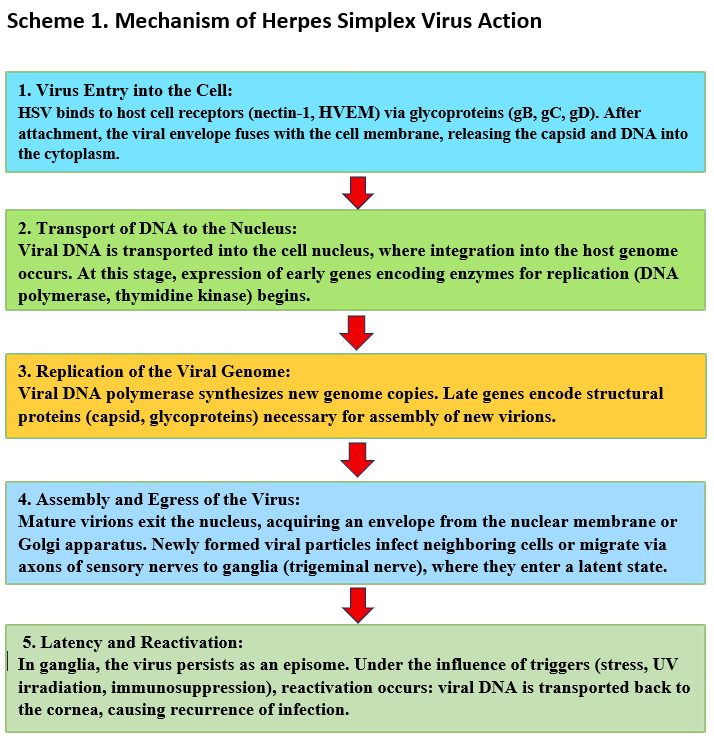
Chapter 1: Etiology and Pathogenesis of Herpetic Keratitis
Herpetic keratitis (HK) is one of the most common and severe infectious diseases of the cornea caused by the Herpes Simplex Virus (HSV). It develops as a result of the complex interaction between the virus, corneal epithelium, the host immune system, and latent reservoirs of infection. Understanding the etiology and pathogenesis of HK is essential for diagnosis, therapy, and prevention of its complications.
Mechanisms of HSV Infection
1. Routes of Transmission
The Herpes Simplex Virus is transmitted mainly through contact with mucous membranes or damaged skin. The routes of infection depend on the viral type (HSV-1 or HSV-2) and patient age:
• Contact-Domestic Route (mainly for HSV-1):
— Infection occurs via direct contact with affected skin, mucous membranes, or saliva of an infected person.
— Auto-inoculation is possible by transferring the virus from the lips, nose, or other areas to the eye via hands, towels, contact lenses, or cosmetics.
• Sexual Route (typical for HSV-2):
— Virus is transmitted sexually, which less frequently causes ophthalmic herpes but can lead to neonatal infections.
• Vertical Transmission:
— HSV-2 is transmitted from mother to child during childbirth. In newborns, herpetic keratitis is often accompanied by generalized infection.
• Rare Routes:
— Aerosol transmission (possible in laboratory or medical settings).
— Blood transfusion (in exceptional cases).
2. Primary Infection
Primary infection occurs upon the first contact with the virus.
Viral entry:
HSV enters through microtraumas of skin or mucous membranes, binding to specific receptors (nectin-1, HVEM) on the cell surface.
Once inside, the virus releases DNA into the host cell nucleus where viral replication begins.
Clinical features:
Most patients experience a subclinical primary infection without pronounced symptoms.
Ophthalmic manifestations may include acute epithelial keratitis or herpetic conjunctivitis.
Immune response:
Primary infection stimulates innate immunity. Type I interferons (IFN-α, IFN-β) inhibit viral replication, and neutrophils and macrophages destroy infected cells.
After the acute phase, the virus migrates along sensory nerve endings to regional ganglia, where latency is established.
3. Latency and Reactivation
HSV latency is a key mechanism enabling lifelong viral persistence.
Latent infection:
Viral DNA is maintained in the nucleus of trigeminal nerve neurons as an episome, not integrated into host DNA.
Latency is supported by expression of non-coding transcripts (Latency-Associated Transcripts, LAT) that suppress viral replication and block apoptosis of infected neurons.
Triggers of Reactivation:
HSV reactivates under factors disrupting immune control:
• Physical: ultraviolet radiation, eye trauma, surgical interventions.
• Immunological: immunosuppression (e.g., HIV, chemotherapy), viral or bacterial infections.
• Emotional: stress, fatigue.
• Hormonal: hormonal shifts in women, menstruation.
Pathogenesis of HSV Keratitis
1. Corneal Infection (Primary Infection)
The virus penetrates the corneal epithelial cells, causing their destruction:
Viral replication:
• In infected cells, the virus utilizes host mechanisms for reproduction.
• New viral particles are released, infecting neighboring cells.
Cellular damage:
• The cytotoxic effect of the virus leads to the death of epithelial cells and formation of characteristic dendritic ulcers.
Role of the immune system:
• Local activation of innate immunity causes influx of neutrophils and release of pro-inflammatory cytokines (IL-1, TNF-α).
• Immune mechanisms protect against systemic viral spread but may cause additional tissue damage.
2. Chronic Inflammation (Stromal Keratitis)
During reactivation, the virus affects deeper layers of the cornea:
• Immune response:
— Reactivation activates T cells, which infiltrate the stromal layers and trigger production of pro-inflammatory cytokines (IFN-γ, IL-17).
— Antibodies against viral antigens may cause complement-dependent stromal damage.
• Neovascularization:
— Chronic inflammation stimulates growth of blood vessels within the cornea, disrupting its transparency.
• Scarring:
— Fibrosis of the stroma due to inflammation leads to persistent reduction of visual acuity.
3. Endothelial Lesions and Keratouveitis
In severe recurrences, the deep corneal layers and anterior chamber are involved:
• Endothelial keratitis:
— Stromal edema and endothelial damage impair corneal hydration.
• Keratouveitis:
— Anterior chamber inflammation may be accompanied by elevated intraocular pressure and secondary glaucoma development.
Modern Concepts of Pathogenesis
See Appendix (QR code linking to Source No.7)
1. Role of Genetic Predisposition:
• Genetic polymorphisms in immune response genes such as TLR3 and IFNL3 may increase the risk of severe herpetic keratitis.
2. Corneal Microbiome:
• Alterations in the ocular surface microbiota are associated with frequent recurrences.
3. Epigenetic Mechanisms:
• Studies indicate that epigenetic changes in trigeminal nerve neurons can modulate HSV latency and reactivation.
Conclusion
The etiology and pathogenesis of herpetic keratitis involve complex interactions among viral factors, immune response, and host conditions. Deep understanding of these processes allows development of new approaches for treatment and prevention, minimizing recurrence risk and long-term complications.
Stages of the Pathogenesis of HSV Keratitis
Herpetic keratitis is a stepwise pathological condition caused by features of the life cycle of Herpes Simplex Virus (HSV) and the immune system’s response. The pathogenesis is divided into three key phases: latent infection, reactivation, and tissue damage. Each phase is driven by complex interactions between viral factors and host defense mechanisms.
1. Latent Infection
After the primary infection, HSV travels through sensory nerves to regional ganglia where it enters latency.
A) Process of Transition to Latency:
• Virus migration to ganglia:
— After infecting the cornea, the virus is transported via axonal flow into sensory neurons, predominantly the trigeminal nerve (ganglion trigeminale).
— Here, viral DNA remains as an episome, not integrated into the host cell genome.
• Mechanisms of latency:
— During latency, only certain non-coding RNAs called Latency-Associated Transcripts (LAT) are expressed. These transcripts:
• Suppress viral replication.
• Block apoptosis of infected neurons.
• Reduce immune activity around the ganglion.
•Epigenetic modifications of viral DNA play a critical role in regulating its transcription during latency.
B) Immune Control of Latent Infection:
• Latency is maintained by immune surveillance primarily through CD8+ T-cells residing in the ganglia.
• Effector cytokines such as interferon-gamma (IFN-γ) create a microenvironment that inhibits viral reactivation.
2. Reactivation: Triggers and Mechanisms
In the latent state, under certain triggers, the virus reactivates leading to active replication and migration back to the corneal tissues.
A) Major Triggers of Reactivation:
Stress:
• Emotional or physical overexertion activates the hypothalamic-pituitary-adrenal axis, leading to the release of glucocorticoids.
• Stress hormones weaken immune control and increase the likelihood of viral reactivation.
Ultraviolet Radiation (UV):
• UV rays induce localized inflammation and tissue damage.
— There is increased production of pro-inflammatory cytokines such as IL-1 and IL-6, which reduce the activity of immune cells controlling the virus.
• UV radiation also activates MAPK signaling pathways that promote viral replication.
Immunosuppression:
• Immunodeficiency states (e.g., HIV infection, malignancies, chemotherapy) impair cellular immunity, creating conditions for viral reactivation.
• The use of systemic corticosteroids or other immunosuppressants significantly raises the risk of reactivation.
B) Mechanism of Reactivation:
• Reactivation is initiated by suppression of immune control within ganglia, leading to activation of viral replication.
• The active virus migrates back along sensory nerve endings to the cornea, causing tissue damage.
3. Tissue Damage: Viral and Immune Contributions
Tissue destruction in HSV keratitis is caused by two main mechanisms: direct cytotoxic effects of the virus and immune-mediated injury.
A) Cytotoxic Effect of the Virus:
1. Epithelial Cell Destruction:
• HSV replicates in corneal epithelial cells, leading to cell death via necrosis and apoptosis.
• Dendritic and geographic ulcers form as a result of focal epithelial degeneration.
2. Viral Protein Activity:
• The virus encodes proteins that suppress immune responses, such as ICP47, which inhibits antigen presentation on MHC-I.
• US3 and UL41 proteins protect infected cells from apoptosis, prolonging their viability for viral replication.
B) Immune Response as a Source of Damage:
Role of Innate Immunity:
• Neutrophils and macrophages recruited to the infection site release reactive oxygen species and pro-inflammatory cytokines (IL-1, TNF-α).
• This enhances inflammation but also causes collateral tissue damage.
Adaptive Immune Response:
• CD4+ and CD8+ T lymphocytes infiltrate stromal layers of the cornea, provoking chronic inflammation.
• Overactivation of T cells, especially TH1 and TH17 subsets, leads to production of interferon-gamma (IFN-γ) and IL-17, intensifying inflammation.
Fibrosis and Neovascularization:
— Chronic inflammation stimulates corneal neovascularization and fibrous tissue formation, disrupting transparency and reducing vision.
C) Final Pathology:
— In severe recurrences, inflammation may spread to deeper ocular structures (e.g., anterior chamber) causing keratouveitis, increased intraocular pressure, and secondary glaucoma development.
Modern Concepts of Tissue Damage
Genetic Predisposition:
— Polymorphisms in genes such as IL-1B and IFNL3 are associated with the severity of inflammation.
Role of the Microbiota:
— Alterations in the ocular surface microbiome influence the frequency and intensity of recurrences.
Epigenetics:
— Epigenetic modifications of viral and host cellular DNA may determine susceptibility to reactivation.
Conclusion:
The staged pathogenesis of HSV keratitis highlights the importance of viral latency and reactivation triggers as key factors governing recurrences and disease severity. The combination of direct cytotoxic viral effects and an excessive immune response shapes the clinical picture, necessitating a balanced therapeutic approach.
Types of Corneal Involvement in Herpetic Keratitis
Herpetic keratitis (HK) is characterized by a wide spectrum of corneal lesions, ranging from superficial epithelial to deep endothelial and stromal involvement. Each lesion type correlates with distinct pathogenetic mechanisms involving viral replication, inflammatory responses, and immune dysregulation.
1. Epithelial Keratitis: Role of Viral Replication
Epithelial keratitis is the earliest and most common corneal involvement in herpetic infection, caused by active replication of herpes simplex virus within epithelial cells.
Pathogenesis:
1. Viral Entry:
— HSV infects the corneal epithelium by binding to cellular receptors such as nectin-1 and HVEM (herpesvirus entry mediator). Upon entry, viral DNA is released into the host cell nucleus where active replication begins.
2. Cellular Destruction:
— Viral replication leads to accumulation of viral particles intracellularly followed by cell lysis.
— Characteristic dendritic or geographic ulcers form, visualized with fluorescein staining.
3. Inflammatory Response:
— Innate immunity is triggered, leading to secretion of interferons (IFN-α, IFN-β) and recruitment of neutrophils.
— Local inflammation limits viral replication but may also cause additional epithelial cell damage.
Clinical Manifestations
• Typical symptoms include pain, photophobia, foreign body sensation, tearing, and decreased vision.
• Biomicroscopic examination reveals branch-like dendritic lesions with blister-like edges filled with viral particles.
Treatment Features
• The primary approach involves antiviral therapy (e.g., topical acyclovir or ganciclovir препараты).
• Avoidance of corticosteroids at this stage is crucial, as they can enhance viral replication.
2. Stromal Keratitis: Autoimmune Reactions and Fibrosis
Stromal keratitis occurs with involvement of the deeper layers of the cornea, often as a result of viral reactivation or autoimmune dysregulation. It is the most destructive form of herpetic keratitis, capable of causing irreversible structural changes in the cornea.
Pathogenesis:
1. Initiation of Inflammation:
• Stromal keratitis is not always associated with active viral replication; instead, immune mechanisms initiated by viral antigens are at the core.
• Expression of viral proteins in stromal cells provokes an inflammatory response, attracting T cells and macrophages.
2. Autoimmune Component:
• Reactive T cells (especially TH1 and TH17) attack stromal tissues, perceiving them as foreign.
• Secretion of cytokines such as IFN-γ and IL-17 enhances inflammation and causes degradation of the extracellular matrix.
3. Fibrosis:
• Chronic inflammation stimulates fibroblasts to produce excess collagen, leading to scar tissue formation.
• Neovascularization of the cornea, driven by inflammation, impairs transparency.
Clinical Manifestations
• Patients complain of progressive visual acuity reduction, photophobia, and pain.
• Biomicroscopy reveals stromal edema, infiltrates, and scarring.
Treatment Features
• Combined therapy with antiviral agents and topical corticosteroids (to control inflammation).
• Immunosuppressants such as cyclosporine may be used for severe forms.
3. Endothelial Keratitis: Endothelial Dysfunction
Endothelial keratitis (disciform keratitis) is a deep corneal lesion characterized by inflammation of the endothelium and stroma, leading to marked impairment of its transparency.
Pathogenesis:
1. Viral Reactivation in the Endothelium:
• Viral antigens or particles activate localized inflammation in endothelial cells.
• Direct viral infection of the endothelium is rarer; damage is more commonly immune-mediated.
2. Immune Inflammation:
• Circulating T cells and monocytes infiltrate the endothelium, provoking dysfunction.
• Release of proinflammatory cytokines (e.g., TNF-α, IL-6) causes corneal and stromal edema.
3. Endothelial Dysfunction:
• The endothelium loses its ability to effectively regulate corneal hydration.
• Pronounced stromal edema develops, significantly reducing corneal transparency and visual acuity.
Clinical Manifestations
• Patients report rapid vision deterioration associated with corneal edema.
• Biomicroscopy reveals disc-shaped edema and endothelial precipitates.
Treatment Features
• Antiviral drugs combined with corticosteroids are used to control inflammation.
• In cases of refractory edema, hyperosmotic agents (e.g., sodium chloride solutions) may be required.
General Remarks on Lesion Types
• Progression Between Forms: Corneal lesions may progress from epithelial to stromal and endothelial keratitis, necessitating timely intervention.
• Chronic Changes: Stromal fibrosis and neovascularization are irreversible, emphasizing the importance of early diagnosis and treatment.
• Immunomodulation: Modern therapeutic approaches include immunotherapy aimed at reducing autoimmune damage without compromising antiviral defense.
Characteristics of pathogenesis, clinical manifestations, and treatment of various herpetic keratitis forms are summarized in Table 1.
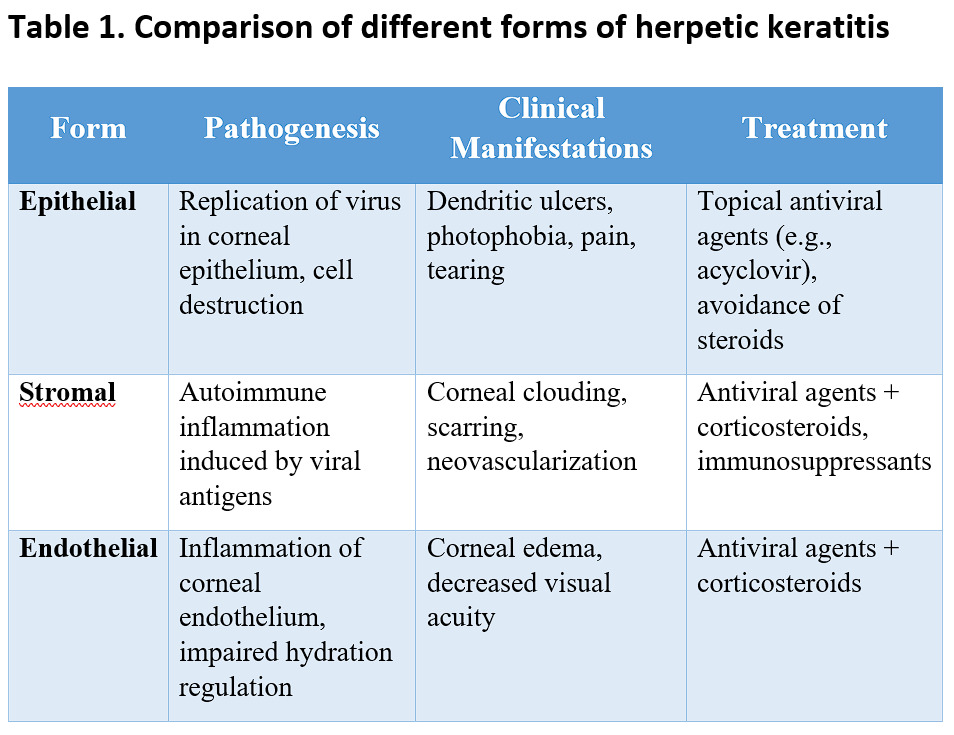
Conclusion:
The types of corneal lesions in herpetic keratitis illustrate the complex interplay between viral and immune factors. Effective treatment requires an individualized approach based on disease stage and the predominant pathogenetic mechanism.
Chapter 2: Clinical Presentation and Classification of Forms
Herpetic keratitis (HSV keratitis) displays a variety of clinical forms that differ in the depth of corneal involvement, pathogenetic mechanisms, and prognosis. The classification includes epithelial, stromal (necrotic and interstitial), endothelial, and metaherpetic keratitis. Each form has distinct clinical and diagnostic features important for timely diagnosis and treatment.
1. Epithelial Herpetic Keratitis
Clinical Manifestations:
• Patients complain of acute decrease in visual acuity, foreign body sensation, photophobia, tearing, and mild pain.
• Typical lesions include:
— Dendritic ulcers: branching linear lesions of the corneal epithelium with typical bulbous endings.
— Geographic ulcers: larger, irregularly shaped lesions formed by coalescing dendritic patches.
Pathogenesis:
• Associated with active HSV replication in epithelial cells leading to their destruction and inflammation.
• The viral component predominates, with minimal immune response at this stage.
Diagnostic Features:
— Biomicroscopy with fluorescein staining reveals ulcers with bright fluorescence.
— Confocal microscopy shows the presence of viral particles and inflammatory cells.
Prognosis and Complications:
• With timely therapy, the process usually remains confined to the corneal surface without deep tissue damage.
• Without treatment, progression to stromal keratitis may occur.
2. Stromal Herpetic Keratitis
Classification:
• Necrotizing Stromal Keratitis:
— Less common, characterized by direct viral infection of stromal cells with massive inflammation.
— Accompanied by pronounced corneal tissue necrosis, neutrophilic infiltration, and edema.
— Frequently leads to scar formation, corneal thinning, and perforation.
• Interstitial Stromal Keratitis:
— Immune-mediated lesion without active viral replication.
— Driven by immune reactions against viral antigens or autoantigens.
— Characterized by chronic stromal inflammation with infiltration of lymphocytes, plasma cells, and macrophages.
Clinical Manifestations:
• Visual acuity reduction, pain, photophobia.
• Biomicroscopy reveals infiltration foci, stromal edema, and corneal haze.
• Corneal neovascularization may occur.
Prognosis:
— Stromal scarring and neovascularization often lead to irreversible loss of corneal transparency and necessitate corneal transplantation.
3. Endothelial Herpetic Keratitis (Disciform Keratitis)
Clinical Manifestations:
• Patients experience a gradual decrease in vision due to corneal edema.
• Biomicroscopy reveals:
— Localized disc-shaped edema.
— Cellular precipitates on the endothelium.
— No significant changes in the epithelium or stroma.
Pathogenesis:
• Occurs due to inflammation associated with immune-mediated attack on endothelial cells.
• Immune complexes and cytokines disrupt the endothelial barrier function leading to impaired corneal hydration.
Prognosis:
• With adequate treatment using anti-inflammatory drugs (corticosteroids) and antiviral therapy, improvement is often observed.
• Chronic forms can lead to persistent corneal opacity.
4. Metaherpetic (Trophic) Keratitis
Clinical Manifestations:
• Characterized by a chronic epithelial defect that fails to heal over a prolonged period despite resolution of active viral infection.
• Patients report persistent vision decrease, photophobia, pain, and tearing.
Pathogenesis:
• Results from impaired epithelial regeneration and altered corneal trophic support.
Main Mechanisms:
• Damage to the basal membrane and limbal stem cells.
• Decreased corneal sensitivity (neuropathic epitheliopathy).
• Chronic inflammation hindering tissue repair.
Clinical Features:
• Extensive epithelial defects with irregular edges.
• Development of vascularization, thinning, and scarring of the cornea.
Prognosis:
• This keratitis form is difficult to treat and often requires surgical intervention, including corneal transplantation.
Key Diagnostic Aspects of HSV Keratitis Forms
• Visualization: Biomicroscopy using fluorescein or rose bengal staining to detect superficial defects.
• Confocal Microscopy: Identification of deep lesions such as endothelial precipitates and stromal infiltrates.
• Laboratory Diagnostics: Polymerase chain reaction (PCR) for viral DNA identification; cytology for detecting giant cells using the Тцанка method.
Conclusion:
Classification of HSV keratitis forms highlights the diversity of lesions from superficial epithelial to deep structural changes. Early diagnosis and accurate determination of the disease form are key to successful treatment and prevention of irreversible complications.
Characteristic Signs of HSV Keratitis
Patient Complaints
Clinical presentation varies with corneal lesion depth and disease form, but some typical complaints can guide diagnosis:
1. Photophobia:
• Caused by receptor irritation in the cornea and increased sensitivity of inflamed tissues to light.
• Patients report discomfort even under moderate lighting.
2. Visual acuity reduction:
• Linked to corneal optical changes including epithelial defects, stromal edema, infiltrates, and scarring.
• Severity depends on lesion location and extent.
3. Ocular pain:
• Ranges from mild to severe depending on disease form.
• Usually caused by superficial corneal inflammation, intensifying with deeper involvement.
4. Foreign body sensation:
• Related to epithelial defects and nerve ending irritation in the cornea.
• Can be accompanied by excessive blinking and reflex tearing.
5. Tearing and Discharge:
• Patients often report clear ocular discharge.
• In bacterial superinfection, discharge may become mucopurulent.
Objective Findings
Using slit-lamp biomicroscopy, key signs of HSV keratitis can be identified that aid in differentiating disease forms:
1. Epithelial Signs:
• Dendritic ulcers:
— The most characteristic feature of epithelial keratitis.
— Branching shape with thickened ends often containing active viral particles.
— Well visualized with fluorescein staining (fluorescence) or rose bengal.
• Geographic ulcers:
— Larger, irregularly shaped defects resulting from coalescence of dendritic ulcers.
— Indicative of progression of epithelial damage.
2. Subepithelial Infiltrates:
• Located beneath the superficial corneal layer and associated with immune response to viral antigens.
• Common in stromal and mixed keratitis types.
• Appear as corneal opacities with localized loss of transparency.
3. Keratoprecipitates (KP):
• Small inflammatory cell deposits on the corneal endothelium.
• Indicative of deep corneal involvement (endothelial keratitis).
• Color and structure vary:
• Fresh KP are small, white, and soft.
• Chronic KP are larger, pigmented, and dense.
4. Corneal Edema:
• Can be localized (in endothelial keratitis) or diffuse (in severe forms).
• Associated with reduced transparency and increased corneal thickness measured by pachymetry.
5. Vascular Invasion (Neovascularization):
• Common in chronic stromal keratitis.
• New vessels invade the corneal stroma disrupting its transparency.
6. Thinning and Scarring:
• Typical of severe and recurrent forms.
• Leads to structural deformities such as keratoconus or perforation.
Additional Objective Signs in Complications
1. Reduced Corneal Sensitivity:
— Often observed in chronic forms.
— Diagnosed with esthesiometry.
— Linked to nerve ending damage caused by HSV neurotrophic effects.
2. Chronic Limbal Inflammation:
— Indicates involvement of corneal epithelial stem cells in the pathological process.
— Manifests as hyperemia and tissue infiltration around the limbus.
Differential Signs
1. Epithelial Keratitis:
— Predominantly viral component.
— Characterized by dendritic or geographic ulcers.
2. Stromal Keratitis:
— Immune-mediated lesion with stromal infiltrates.
— Often associated with neovascularization.
3. Endothelial Keratitis:
— Localized corneal edema and presence of keratoprecipitates on the endothelium.
— Minimal epithelial changes.
4. Metaherpetic Keratitis:
— Presence of chronic epithelial defect with reduced regeneration and corneal vascularization.
Practical Significance
Accurate interpretation of symptoms and objective findings allows rapid diagnosis of HSV keratitis and determination of its form. This is critical for therapy selection, as different clinical manifestations require varied treatment approaches.
Differential Diagnosis of HSV Keratitis
Differential diagnosis plays a crucial role in timely application of appropriate treatment strategies, as several infectious agents may present with similar clinical signs. The main tasks are distinguishing HSV keratitis from other viral, bacterial, fungal infections, and autoimmune diseases.
1. HSV Keratitis vs. VZV Keratitis
Varicella-Zoster Virus (VZV), the cause of chickenpox and shingles, is also a herpesvirus but induces clinically distinct keratitis forms compared with HSV. Differentiating these infections requires thorough clinical assessment and laboratory data.
Clinical Differences:
1. Lesion Localization:
• HSV can affect one or both eyes, most commonly involving central and peripheral cornea.
• VZV primarily causes unilateral involvement, often affecting lateral corneal areas. VZV keratitis is frequently associated with herpetic dermatitis presenting as vesicles, often in the region of the ophthalmic nerve.
2. Lesion Character:
• HSV typically features dendritic or geographic ulcers and subepithelial infiltrates.
• VZV shows more pronounced stromal infiltrates which may be necrotic and often accompanied by marked neovascularization.
3. Associated Symptoms:
• HSV lacks significant cutaneous inflammation and skin vesicles; patients may not exhibit skin manifestations in herpes recurrence.
• VZV keratitis commonly co-occurs with herpetic dermatitis characterized by vesicular eruptions in the orbital region.
4. Patient Age:
• HSV occurs at all ages but is more frequent in children and young adults.
• VZV usually affects older adults or immunocompromised individuals.
Diagnosis:
• PCR and Virological Testing: Polymerase Chain Reaction (PCR) is essential for precise differentiation by detecting viral DNA. VZV possesses distinct genetic markers differentiating it from HSV.
• Dermatological Examination: Identifying skin vesicular lesions is critical for VZV diagnosis.
2. Differentiation from Bacterial Keratitis
Bacterial corneal infections can also present with ulcers and infiltrates, mimicking herpetic keratitis. However, there are several key differences aiding differential diagnosis.
Clinical Differences:
1. Onset Time:
• HSV: Often begins with eye redness and pain, developing over several days, followed by ulcer formation.
• Bacterial keratitis: Usually progresses rapidly with severe symptoms — intense pain, swelling, hypopyon (pus in anterior chamber).
2. Ulcer Characteristics:
• HSV: Dendritic and geographic ulcers with characteristic edges and limited inflammatory reaction.
• Bacterial keratitis: Ulcers have rough, irregular margins with severe edema and often prominent purulent exudate. Fibrinous deposits frequently surround the ulcer.
3. Associated Symptoms:
• HSV: Moderate inflammation signs such as photophobia, with possible aftereffects.
• Bacterial keratitis: Severe systemic symptoms including fever and general malaise. Marked ocular pain and swelling.
Diagnosis:
Бесплатный фрагмент закончился.
Купите книгу, чтобы продолжить чтение.